Abstract
Purpose
We compared the efficacy of applying mydriatics to the conjunctiva of the lower eyelid by using a cotton-tipped applicator with an eye-dropping method.
Methods
Thirty children under 16 years of age (60 eyes) and 30 adults more than 20 years old (60 eyes) were randomly chosen. Mydriatics were applied to each eye using an eye-dropping method for one eye and a conjunctival application method using a cotton-tipped applicator for the other eye. Pupil size was measured before applying mydriatics, immediately and at 30 and 60 minutes after application. Also, we investigated the degree of discomfort.
Results
In the children, the pupil size in the eye dropping group and the conjunctival application group were each 8.01±0.57 mm and 7.97±0.57 mm at 60 minutes after applying mydriatics (P=0.07). In the adults, the pupil size was 7.94±0.59 mm in the eye-dropping group and 7.89±0.55 mm in the conjunctival application group at 60 minutes after applying mydriatics (P=0.074). In terms of degree of discomfort, the adults found the conjunctival application method to be significantly more unpleasant than the eye-dropping method at each three applications (P=0.001, 0.001, 0.001).
Conclusions
The conjunctival application method using a cotton-tipped applicator has an equal effect in terms of mydriasis compared to the eye-dropping method. It is convenient to use in children and in the elderly who show less compliance. Also, this new method reduces systemic absorption of the medication. Therefore, the conjunctival application method is a good substitute for the conventional methods for mydriasis.
References
1. Elibol O, Alcelik T, Yuksel N, Caglar Y. The influence of drop size of cyclopentolate, phenylephrine and tropicamide on pupil dilatation and systemic side effects in infants. Acta Ophthalmol Scand. 1997; 75:178–80.

2. Jones LW, Hodes DT. Possible allergic reactions to cyclopentolate hydrochloride: Case reports with literature review of uses and adverse reactions. Ophthalmic Physiol Opt. 1991; 11:16–21.

3. Sato EH, de Freitas D, Foster CS. Abuse of cyclopentolate hydrochloride drops. N Eng J Med. 1992; 326:1363–4.
4. Tripathi SK, Mondal TK. Systemic toxicity with cyclopentolate eye drop. J Indian Med Assoc. 1990; 88:266.
5. Awan KJ. Systemic toxicity of cyclopentolate hydrochloride following topical ocular instillation. Ann Ophthalmol. 1976; 8:803–6.
6. Khurana AK, Ahluwalia BK, Rajan C, Vohra AK. Acute psychosis associated with topical cyclopentolate hydrochloride. Am J Ophthalmol. 1988; 105:91.

7. Bartlett JD, Wesson MD, Swiatocha J, Woolley T. Efficacy of a pediatric cycloplegic administered as a spray. J Am Optom Assoc. 1993; 64:617–21.
8. Ismail EE, Rouse MW, De Land PN. A comparison of drop instillation and spray application of 1% cyclopentolate hydrochloride. Optom Vis Sci. 1994; 71:235–41.

9. Wesson MD, Bartlett JD, Swiatocha J, et al. Mydriatic efficacy of a cycloplegic spray in the pediatric population. J Am Optom Assoc. 1993; 64:637–40.
10. Lahdes KK, Huupponen RK, Kaila TJ. Ocular effects and systemic absorption of cyclopentolate eyedrops after canthal and conventional application. Acta Ophthalomol. 1994; 72:198–702.

11. Stolovitch C, Alster Y, Goldstein M, et al. Application of cyclopentolate 1% to the medial canthus in children. J Pediatr Ophthalmol Strabismus. 1998; 35:182–4.

Figure 1.
Comparison of the unfavorable scale score according to the refusal response in children (n=30).
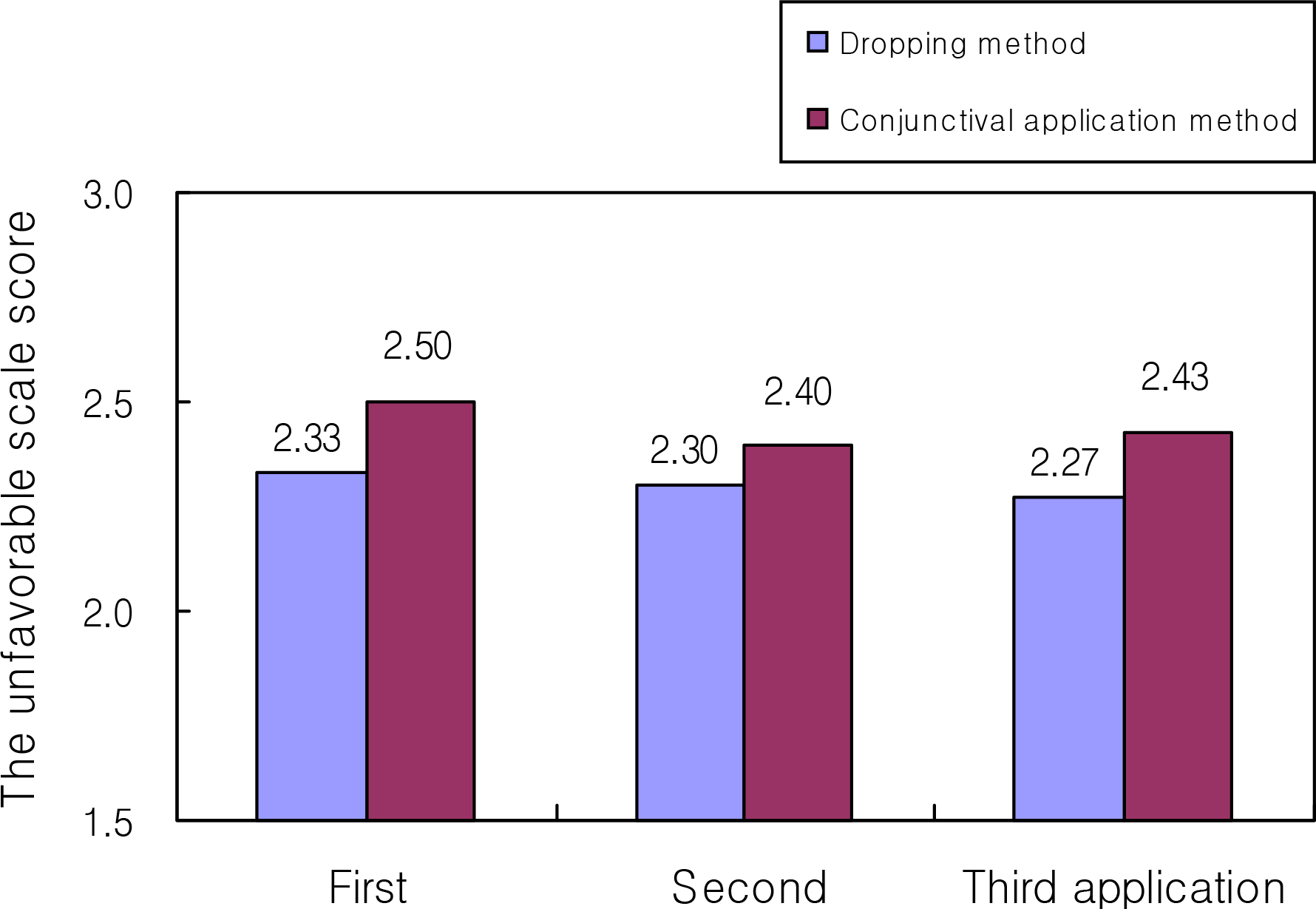
Figure 2.
Comparison of the unfavorable scale score according to the questionnaire in adults (n=30).
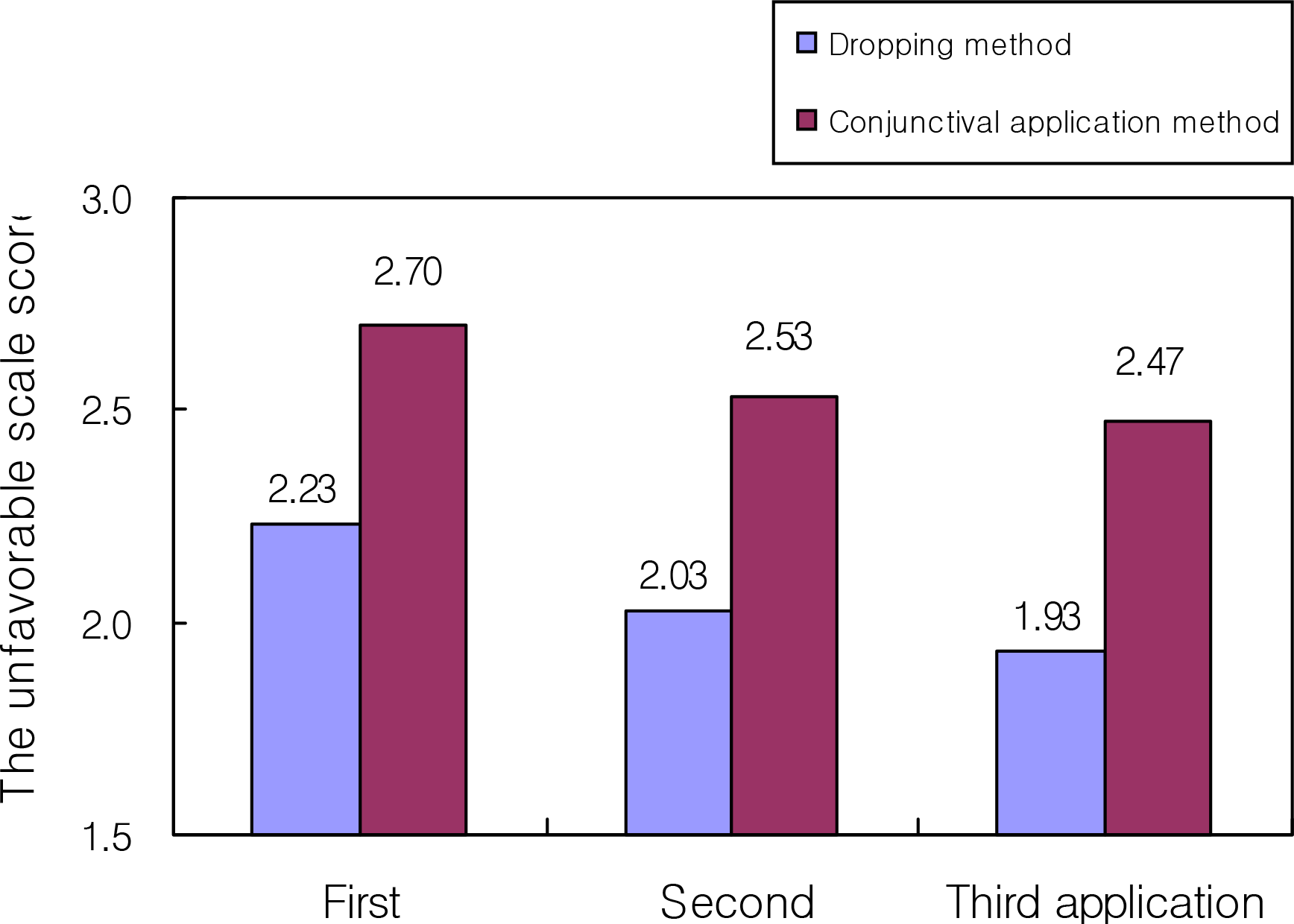
Table 1.
Characteristics of patients
| | Children less than 16 years of age | Adults more than 20 years of age |
|---|---|---|
| No. of patients (eyes) | 30 (60) | 30 (60) |
| Age (years old) | 7.4±2.0 (4–14) | 62.4±12.8 (30–82) |
| Gender (Male: Female) | 17: 13 | 17: 13 |
| Ocular disease (No. of eyes) | | |
| DM* retinopathy | 0 | 4 |
| Blepharospasm | 0 | 2 |
| Systemic disease (No. of patient) | | |
| DM* | 0 | 8 |
| Hypertension | 0 | 6 |
| Cervical arthritis | 0 | 1 |
Table 2.
Comparison of the pupil size (mm) between the dropping method and The conjunctival application method using cotton-tipped applicator in children (n: number of eyes)
| | Pupil size | P-value | |
|---|---|---|---|
| | Dropping method (n=30) | Conjunctival application method (n=30) | (<0.05) |
| Before applying mydriatics | 6.03±0.51 | 6.04±0.51 | 0.08 |
| Immediate after applying mydriatics* | 6.96±0.69 | 6.79±0.74 | 0.003 |
| 30 min after applying mydriatics | 7.75±0.63 | 7.59±0.67 | 0.002 |
| 60 min after applying mydriatics | 8.01±0.57 | 7.97±0.57 | 0.07 |
Table 3.
Comparison of the pupil size (mm) between the dropping method and The conjunctival application method using cotton-tipped applicator in adults (n=number of eyes)
| | Pupil size | P-value | |
|---|---|---|---|
| | Dropping method (n=30) | Conjunctival application method (n=30) | (<0.05) |
| Before applying mydriatics | 4.08±0.85 | 4.07±0.84 | 0.286 |
| Immediate after applying mydriatics* | 5.66±0.87 | 5.51±0.90 | 0.001 |
| 30 min after applying mydriatics | 7.66±0.66 | 7.49±0.74 | 0.001 |
| 60 min after applying mydriatics | 7.94±0.59 | 7.89±0.55 | 0.074 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download