Abstract
Radiofrequency ablation (RFA) is a minimally invasive procedure that has been considered as a relatively safe treatment for patients with small hepatocellular carcinoma (HCC). However, RFA has been shown to be associated with complications including mechanical and thermal damage. A 74-year-old man with hepatitis C virus-associated HCC was admitted to our hospital. Abdominal computed tomography revealed two lobulated-HCC in segments 4 and 5. He had no medical history of hypertension and cardiac disease. During RFA, blood pressure was elevated to 200/140 mmHg. There was no evidence of pulmonary embolism, aortic dissection, or ischemic heart disease. Laboratory findings for catecholamine surge were all within normal limits. After continuous intravenous nitroglycerin and oral beta-blocker treatment, patient's blood pressure gradually decreased and back within the normal range. Hypertensive crisis after RFA treatment for HCC is rare. Most reported cases of hypertensive crisis during RFA were related to adrenal gland injury with a release of catecholamine. In our case, the site of HCC was not close to the adrenal gland, and there was no evidence of catecholamine surge. Herein, we report a very rare case of hypertensive crisis without a surge in adrenal hormones after RFA treatment for HCC.
Hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor, which frequently occurs in the setting of cirrhosis. Surgical resection is the best curative treatment option.1 Nonetheless, there are several limitations to surgical resection, including tumor location, number, hepatic reserve, and patient performance status.2 Therefore, alternative therapeutic options, such as percutaneous ethanol injection (PEI), radiofrequency ablation (RFA), and transarterial chemoembolization (TACE) have been proposed and used to treat HCC.34 RFA is a minimally invasive procedure, which has been considered as a relatively simple and safe modality in treating cirrhotic patients with small unresectable HCC. However, RFA may cause early and late complications related to mechanical and thermal damages. Injury to the adjacent normal tissue is a major concern during RFA of HCC. Bowel perforation, cholecystitis, bile duct stricture, liver abscess, pleural effusion, pneumothorax, pulmonary embolism, intraperitoneal bleeding, and portal vein thrombosis have been reported.5678 Hypertensive crisis related to unintended adrenal gland injury during RFA of liver tumor has also been reported.9 We experienced a very rare case of hypertensive crisis after RFA for HCC without evidence of adrenal gland injury and dramatic surge in several adrenal hormones. Herein, we report clinical course and management of this rare case.
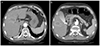
A 74-year-old man with HCC was referred to our hospital. Tumors were discovered during a routine follow-up abdominal sonography in another hospital. Computer tomography (CT) revealed a 2.2-cm lobulated HCC in segment 5 and 1.0-cm HCC in segment 4 (Fig. 1). The image of tumors showed heterogenous enhancement in the arterial phase and washout pattern in both the portal and delayed phases. He had a medical history of liver cirrhosis, secondary to chronic hepatitis C, no history of hypertension and cardiac disease. There was no specific past medical history. He was recommended from the other hospital to undergo RFA for both HCCs. Physical examination and laboratory findings showed mild anemia (hemoglobin level was 10.9 g/dL), and Child-Turcotte-Pugh score was 6, which was stratified as class A. He was afebrile, with a blood pressure of 120/90 mmHg and a heart rate of 78 beats per minute. The initial electrocardiogram (ECG) showed sinus rhythm and right bundle branch block. Laboratory data revealed a platelet count of 98,000/mm3, aspartate aminotransferase of 19 IU/L, alanine aminotransferase of 3 IU/L, total bilirubin of 0.4 mg/dL, albumin of 3.1 g/dL, prothrombin time of 14.9 sec (78%), and alpha fetoprotein of 19.8 ng/mL. The results of viral markers were HBsAg/anti-HBs (-/-), HCV Ab (+) and HCV RNA count was 18 IU/mL.
Percutaneous RFA was safely performed under local anesthesia and ultrasound guidance. During RFA, blood pressure (BP) was elevated to 200/140 mmHg. After RFA, BP was elevated to 220/120 mmHg and level of oxygen saturation was 82% (Fig. 2). The patient was immediately transferred to the intensive care unit, and continuous intravenous nitroglycerin infusion was started with BP and oxygen saturation monitoring. In the intensive care unit, he had an alert mental status, and there were no symptoms or signs of dyspnea (level of oxygen saturation was 100%). ECG showed sinus rhythm with right bundle branch block, and the heart rate of 80 beats per minute. After continuous intravenous nitroglycerin treatment, his blood pressure was gradually decreased, back within the normal range. A cardiologist recommended to use oral beta-blocker (carvedilol 12.5 mg twice a day).
To evaluate hypertensive crisis, we checked his chest CT, ECG, cardiac enzyme, echocardiacgraphy, serum metanephrine, serum catecholamine (norpinephrine, epinephrine), 24-hour urinary vanillylmandelic acid (VMA), and 24-hour urinary metanephrine. There was no evidence of pulmonary embolism, aortic dissection, and ischemic heart disease. Laboratory findings for catecholamine surge showed normal range of serum metanephrine (0.29 nmol/L; normal, <0.5 nmol/L), serum norepinephrine (104.4 pg/mL; normal, <600 pg/mL), serum epinephrine (34.9 pg/mL; normal, <900 pg/mL), 24-hour urinary VMA (2.4 mg/day; normal, 2.0-7.0 mg/day), and 24-hour urinary metanephrine (0.4 mg/day; normal, <0.8 mg/day). Abdominopelvic CT was performed one day after RFA and showed completed RFA state on S4 and small residual HCC on the posterior margin of RFA site on S5 (Fig. 3).
One day after the initial RFA, BP was controlled with a beta blocker, and his vital signs were stable. He was transferred to a general ward. He was then planned for a second RFA to manage any remnant HCCs on S5. Two days after the initial RFA, the second RFA was performed. There was no sign of hypertensive crisis during and after RFA. He was discharged with hypertension medications and was regularly followed-up at the outpatient clinic.
Hypertensive crisis is defined as a sudden increase in systolic and diastolic blood pressures associated with potential end-organ damage in the central nervous system, heart, or kidneys.10 Causes of hypertensive crisis are highly varied. The most common causes of hypertensive crisis include cerebral infarction, intracranial hemorrhage, pulmonary edema, hypertensive encephalopathy, myocardial infarction, congestive heart failure, aortic dissection, sympathetic crises (pheochromocytoma), eclampsia, and malignant hypertension.
RFA has been reported as a safe procedure, using a high frequency heat that induces electric current to the tissue. This results in protein denaturation and tissue necrosis.11 Most reported cases of hypertensive crisis during RFA were related to adrenal gland injury.1213 Heating and injury of the adrenal tissue may cause a release of catecholamine into the circulation. Catecholamine surge can lead to tachycardia, arrhythmia, and rapid increases in the afterload.
Hypertensive crisis in hepatic RFA is rare. Onik et al.9 reported life-threatening hypertensive crisis in two hepatic RFA patients with a massive increase in catecholamine levels (dopamine was 62,920 pg/mL, epinephrine was 22,240 pg/mL, norepinephrine was 4,440 pg/mL). They suggested that ablation of tumors located in the posterior right lobe of the liver can cause unintended injuries to the adrenal gland that can lead to a release of catecholamine and subsequent hemodynamic effects.9
Recently, You et al. reported a malignant hepatic paraganglioma that mimicked a liver tumor with hypertensive crisis and noradrenaline secretion during hepatic lobectomy.14 However, in the present case, we diagnosed the liver tumors as HCCs using the four-phase dynamic CT findings, which showed to be compatible with a typical HCC of early arterial enhancement followed by a wash-out pattern on the portal and delayed phases. Furthermore, he had HCV-associated cirrhosis and alpha fetoprotein was slightly elevated. Therefore, we did not perform any more studies, such as liver biopsy and dynamic magnetic resonance imaging due to concerns about seeding of tumor along the biopsy tract and high cost.
In the present case, tumor was located on S4 and S5, but not close to the adrenal gland. We measured the serum and urinary adrenal hormones about five to six hours after hypertensive crisis. The values of epinephrine, norepinephrine, metanephrine, and urinary VMA were not elevated. Other presentations of hypertensive crisis were not founded.
Lidocaine hypersensitivity might cause hypertension. However, local anesthesias with lidocaine-induced hypertensive crisis are very rare. Lidocaine with epinephrine changes in vital sign were systolic BP elevation and diastolic BP depression. In our case, both systolic and diastolic BPs were increased. Furthermore, patients had no past medical history of lidocaine anaphylaxis.
We did not prove any evidence of catecholamine surge at the time of hypertensive crisis. The relatively late blood sampling and short half-life of catecholamine (epinephrine, 3.5 minutes; norepinephrine, 2-2.5 minutes; metanephrine, 3-6 minutes) may be one of the causes that does not reflect actual catecholamine surge (hypertensive crisis event time: 16:30, serum catecholamine sampling time: 22:30, serum metanephrine sampling time: next day 11:00).151617 Considering the event time of hypertensive crisis, sampling time, and short half-life time of adrenal hormones, this hypertensive crisis might be caused by an adrenal gland injury. However, the RFA site was not close to the adrenal gland, and absence of hypertensive crisis after the second RFA for any remnant lesions on S5 suggest that hypertensive crisis during and after RFA may occur without adrenal gland injury. Therefore, physicians should be cautious about hypertensive crisis at the time of RFA for HCC, even if it is not located close to the adrenal gland.
Figures and Tables
Fig. 1
Computed tomography image of 2.2-cm lobulated hepatocelluar carcinoma in S5 (white arrow). Early enhancement on arterial phase (A), wash out pattern on portal phase (B), wash out pattern on delayed phase (C).

References
1. Zhou L, Rui JA, Wang SB, et al. Outcomes and prognostic factors of cirrhotic patients with hepatocellular carcinoma after radical major hepatectomy. World J Surg. 2007; 31:1782–1787.
2. McGhana JP, Dodd GD 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001; 176:3–16.
3. Mazzanti R, Arena U, Pantaleo P, et al. Survival and prognostic factors in patients with hepatocellular carcinoma treated by percutaneous ethanol injection: a 10-year experience. Can J Gastroenterol. 2004; 18:611–618.
4. Vogl TJ, Zangos S, Balzer JO, et al. Transarterial chemoembolization (TACE) in hepatocellular carcinoma: technique, indication and results. Rofo. 2007; 179:1113–1126.
5. Curley SA, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004; 239:450–458.
6. Chen TM, Huang PT, Lin LF, Tung JN. Major complications of ultrasound-guided percutaneous radiofrequency ablations for liver malignancies: single center experience. J Gastroenterol Hepatol. 2008; 23(8 Pt 2):e445–e450.
7. Zavaglia C, Corso R, Rampoldi A, et al. Is percutaneous radiofrequency thermal ablation of hepatocellular carcinoma a safe procedure? Eur J Gastroenterol Hepatol. 2008; 20:196–201.
8. Jansen MC, van Duijnhoven FH, van Hillegersberg R, et al. Adverse effects of radiofrequency ablation of liver tumours in the Netherlands. Br J Surg. 2005; 92:1248–1254.
9. Onik G, Onik C, Medary I, et al. Life-threatening hypertensive crises in two patients undergoing hepatic radiofrequency ablation. AJR Am J Roentgenol. 2003; 181:495–497.
10. Varon J, Marik PE. The diagnosis and management of hypertensive crises. Chest. 2000; 118:214–227.
11. Rhim H, Goldberg SN, Dodd GD 3rd, et al. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics. 2001; 21 Spec No:S17–S35. discussion S36-S39.
12. Chini EN, Brown MJ, Farrell MA, Charboneau JW. Hypertensive crisis in a patient undergoing percutaneous radiofrequency ablation of an adrenal mass under general anesthesia. Anesth Analg. 2004; 99:1867–1869. table of contents.
13. Keeling AN, Sabharwal T, Allen MJ, Hegarty NJ, Adam A. Hypertensive crisis during radiofrequency ablation of the adrenal gland. J Vasc Interv Radiol. 2009; 20:990–991.
14. You Z, Deng Y, Shrestha A, Li F, Cheng N. Primary malignant hepatic paraganglioma mimicking liver tumor: a case report. Oncol Lett. 2015; 10:1176–1178.
15. Abboud I, Lerolle N, Urien S, et al. Pharmacokinetics of epinephrine in patients with septic shock: modelization and interaction with endogenous neurohormonal status. Crit Care. 2009; 13:R120.
16. Beloeil H, Mazoit JX, Benhamou D, Duranteau J. Norepinephrine kinetics and dynamics in septic shock and trauma patients. Br J Anaesth. 2005; 95:782–788.
17. Dekkers T, Deinum J, Schultzekool LJ, et al. Plasma metanephrine for assessing the selectivity of adrenal venous sampling. Hypertension. 2013; 62:1152–1157.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download