Abstract
Background/Aims
Portal hypertension (PH) is a syndrome characterized by chronic increase in the pressure gradient between the portal vein and inferior vena cava. Previous studies have suggested an increased frequency of antral elevated erosive gastritis in patients with PH, as well as an etiologic association; however, there has not been any histological evidence of this hypothesis to date. Our aim was to evaluate the histological features found in elevated antral erosions in patients with portal hypertension.
Methods
Sixty-nine patients were included; 28 with and 41 without PH. All patients underwent endoscopy, and areas with elevated antral erosion were biopsied.
Results
In the PH group, 24 patients had inflammatory infiltration with or without edema and vascular congestion, and 4 patients had no inflammation. In the group without PH, all patients showed inflammatory infiltration of variable intensity. There was no statistical significance between the two groups in the presence of Helicobacter pylori. There as a histological similarity between the two groups, if PH patients without inflammation were excluded; however, more edema and vascular congestion were observed in the PH group (p=0.002).
Since the first description of macroscopic changes occurring in the gastric mucosa of patients with portal hypertension (PH), portal hypertensive gastropathy (PHG) remains a challenge for endoscopists in the past 30 years. PHG has a controversial pathophysiology and a highly variable incidence in the literature, accounting for 4% to 80% of PH patients.12345 This wide variation is likely due to the challenges associated with making a correct endoscopic diagnosis, especially for milder forms or those not associated with bleeding.6 The three most accepted and used classifications (McCormack,7 New Italian Endoscopy Club8 and Baveno Consensus9) are still widely debated, but it is clear that the simplest classification is still the most adopted with the highest consensus among endoscopists.10
In medical practice, there is a correlation between endoscopic findings of PHG and the presence of elevated gastric antral erosions in PH patients. The histological relationship between these gastric manifestations has to date not been fully elucidated. Although the endoscopic appearance of erosions is similar between patients with and without PH, the present study aimed to determine whether an increase in the chronic pressure of the portal system causes these erosions in PH patients or if they are a part of the large spectrum of peptic disease.
Consecutive patients presenting elevated antral erosions during endoscopic examination between December 2010 and June 2012, who were referred to the endoscopy unit of Santa Casa de Sao Paulo School of Medical Sciences, were enrolled in this prospective study. This study was approval by the Ethics in Human Research Committee (No. 138/10) and included in the Brazilian Registry of Clinical Trials (No. RBR-5yr2yx-http://www.ensaiosclinicos.gov.br/rg/RBR-5yr2yx/). We included patients of both genders, regardless of age. We excluded the following patients: those who had previous treatment for Helicobacter pylori (H. pylori) eradication, recently used antibiotics, or did not agree to participate in the study.
Overall, 69 patients admitted for dyspeptic complaints were included and divided into two groups: group I, which included 28 patients with PH and group II, which included 41 patients without PH. We apply a questionnaire to clarify the PH etiology, to ascertain whether they had used proton-pump inhibitors, non-steroidal anti-inflammatory drugs, or tobacco, and whether they had been treated for H. pylori infection.
Patients in group I were characterized by the presence of esophageal varices in varying degrees, regardless of prior history of gastrointestinal bleeding or endoscopic treatment of varices. This group included patients with or without PHG.
In group II, patients underwent an ultrasound to identify any degree of liver disease, including homogeneous liver, normal spleen, and portal vein; study requirement for inclusion was for the diameter to be less than 12 mm.
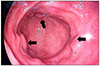
Endoscopic examinations were performed under sedation using midazolam (0.1 mg/kg body weight) and fentanyl (1 µg/kg body weight). Fujinon EPX 2200 videoendoscope (Fujinon Optical CO., Ltd, Tokyo, Japan) was used in conventional videoendoscopy examinations to assess the esophagus, stomach, and duodenum. In group I patients, the presence or absence of PHG was then determined. Gastric biopsies were performed on all patients for H. pylori presence using a urease method; biopsies of antral elevated erosions were also performed (Fig. 1).
During the histopathological analysis, we checked for the presence or absence of inflammation in biopsies to search for factors, such as lymphocytic infiltration, edema, and vascular congestion (Fig. 2). One senior pathologist (MTMF) performed all histologic examinations.
SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Differences were considered statistically significant if p<0.05.
Fisher's exact test was used to assess the differences between groups I and II, regarding the presence of inflammation in the gastric mucosa and to identify whether the presence of H. pylori influenced the inflammatory processes.
Chi-squared test was applied to determine whether there were significant differences between the two groups for the presence of lymphocytic infiltration only and for the presence of lymphocytic infiltration together with edema and vascular congestion.
Table 1 shows the etiologies of PH in group I. H. pylori infection was not an important factor in justifying the presence of inflammatory process in both groups, with no significantly difference (p=0.73) when both groups were compared using Fisher's exact test (Table 2).
In group I (PH, n=28) lymphocytic infiltration alone or associated with edema and vascular congestion were present in 24 patients; however, in four patients there was no sign of lymphocytic infiltration. We excluded these four patients for further analysis. Among the remaining 24 patients with lymphocytic infiltration, 12 belonged to the subgroup without PHG, and 12 patients belonged to the subgroup with PHG. Histopathological evaluation revealed that in the subgroup with PHG, one individual (8.3%) presented only lymphocytic infiltration, and 11 patients (91.7%) presented lymphocytic infiltration, edema, and vascular congestion. In the subgroup without PHG, four (33.3%) presented lymphocytic infiltration alone and eight (66.7%) presented lymphocytic infiltration, edema, and vascular congestion. We did not find any significant differences when comparing the subgroups with and without PHG between those presenting lymphocytic infiltration alone and those presenting the combination of lymphocytic infiltration, edema, and vascular congestion (Table 3).
In group II, 25 patients (61%) presented inflammation with lymphocytic infiltration alone, and 16 patients (39%) presented lymphocytic infiltration with edema and vascular congestion.
Table 3 also shows a comparison between the histological features of group I (excluding the four patients without inflammation) and group II, regarding the presence of lymphocytic infiltration, edema, and vascular congestion.
PHG is a well-established complication of PH. Endoscopic findings range from a fine pinkish speckled pattern to a diffuse hemorrhage. Histological findings include tortuous and dilated vessels in the stomach submucosa and mucosal vascular ectasia concomitant with a few inflammatory signs. This led McCormack et al.7 to suggest the term portal hypertension gastropathy, or PHG, in 1985.
In 1990, D'Amico et al.2 noted that in addition to the vascular changes already described, there were inflammatory changes, especially in mild PHG, whereas they observed architectural distortion with atrophy in more advanced cases of PHG.
In the literature, a few studies have evaluated the accuracy of endoscopic biopsies, mostly showing no correlation between endoscopic and histological findings. Therefore, this cannot be the method that yields a definitive diagnosis of PHG. There is an increased risk of bleeding due to the coagulation profile and platelet dysfunction found in patients with PH.1112 In the present study, despite the increased risk of bleeding in chronic liver disease, no significant bleeding was observed after observing the results of the biopsy.
In 2008, Assef et al.6 showed an increased frequency of elevated erosive gastritis covered with fibrin, affecting the gastric antrum of patients with PH (37.5%). Among these, 16.7% had no endoscopic diagnosis of PHG, while 50% had mild PHG and 33.3% had severe PHG. These findings motivated us to perform a biopsy in an attempt to elucidate how hypertensive gastropathy produces antral lesions.
In practice, we suggest that antral erosions are the primary sign of hypertensive gastropathy in the gastric mucosa because even in the absence of aggressive factors to the antral mucosa, erosions are common in cirrhotic patients. In these biopsies showing erosions, we observed ectasia and edema of the mucosa, with the degree of lymphocytic infiltrate ranging from absent to intense.
These histological features were observed in both patients with and without PH. When comparing these groups, however, it was noted that patients with PH, including both with or without PHG, showed a higher frequency of edema and vascular congestion than those without portal hypertension.
The different prevalence rates of H. pylori infection have been reported depending on the diagnostic method.13 However, we used a histopathological method and a urease test. We observed that the group without PH had a larger number of H. pylori infection compared with the group with PH; but the infection was not significantly associated with inflammatory activity.
We expected to find obvious vascular abnormalities in the biopsies of gastric antral erosions of our samples; however, the findings were variable with respect to inflammation. Despite the limited sample, we found that edema and vascular congestion were more evident in patients with PH. Both patients with and without PH exhibited inflammatory histological changes in antral erosions; therefore, PH in association with PHG should not be considered the sole cause for the erosions observed in these patients.
Thus, although it was endoscopically observed that patients with PH had more antral elevated erosions than patients without PH, it is necessary to conduct follow-up studies with larger samples to determine the cause of hypertensive gastropathy in the gastric antrum. Perhaps this is the main limitation of this study. Meeting the criteria for inclusion in the PHG group and the risk of bleeding in antral biopsies may have reduced our sample number.
The results obtained in this present study that included 69 patients with and without PH demonstrated increased edema and vascular congestion in biopsies of antral elevated erosions from PH patients.
Figures and Tables
Fig. 2
Biopsies of elevated antral erosions in portal hypertensive gastropathy. (A) Edema and vascular congestion (H&E, ×200). (B) Lymphocytic infiltration (H&E, ×400).

References
1. Sarin SK, Sreenivas DV, Lahoti D, Saraya A. Factors influencing development of portal hypertensive gastropathy in patients with portal hypertension. Gastroenterology. 1992; 102:994–999.
2. D'Amico G, Montalbano L, Traina M, et al. Natural history of congestive gastropathy in cirrhosis. The Liver Study Group of V. Cervello Hospital. Gastroenterology. 1990; 99:1558–1564.
3. Calès P, Oberti F, Bernard-Chabert B, Payen JL. Evaluation of baveno recommendations for grading esophageal varices. J Hepatol. 2003; 39:657–659.
4. Kim MY, Choi H, Baik SK, et al. Portal hypertensive gastropathy: correlation with portal hypertension and prognosis in cirrhosis. Dig Dis Sci. 2010; 55:3561–3567.
5. Choe WH. Portal hypertensive gastropathy and gastric antral vascular ectasia. Korean J Gastroenterol. 2010; 56:186–191.
6. Assef MS, Valentino W, Nakamura RK, Camunha MA, Colaiacovo R, Rossini LG. The study of magnifying endoscopy for the diagnosis of portal hypertensive gastropathy. Gastrointest Endosc. 2010; 71:AB379.
7. McCormack TT, Sims J, Eyre-Brook I, et al. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut. 1985; 26:1226–1232.
8. Primignani M, Carpinelli L, Preatoni P, et al. Natural history of portal hypertensive gastropathy in patients with liver cirrhosis. The New Italian Endoscopic Club for the study and treatment of esophageal varices (NIEC). Gastroenterology. 2000; 119:181–187.
9. de Franchis R. Baveno V Faculty. Revising consensus in portal hypertension: report of the baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010; 53:762–768.
10. de Macedo GF, Ferreira FG, Ribeiro MA, Szutan LA, Assef MS, Rossini LG. Reliability in endoscopic diagnosis of portal hypertensive gastropathy. World J Gastrointest Endosc. 2013; 5:323–331.
11. Misra SP, Dwivedi M, Misra V, et al. Endoscopic and histologic appearance of the gastric mucosa in patients with portal hypertension. Gastrointest Endosc. 1990; 36:575–579.
12. Chaves DM, Sakai P, Mucenic M, Iriya K, Iriya Y, Ishioka S. Comparative study of portal hypertensive gastropathy in schistosomiasis and hepatic cirrhosis. Endoscopy. 2002; 34:199–202.
13. Testerman TL, Morris J. Beyond the stomach: an updated view of helicobacter pylori pathogenesis, diagnosis, and treatment. World J Gastroenterol. 2014; 20:12781–127808.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download