Abstract
Background/Aims
DNA double strand break (DSB) is one of the critical types of DNA damage. When unrepaired DSB is accumulated in the nucleus of the cells having mutations in such genes as p53, it will lead to chromosomal instability and further more to mutation of tumor-activating genes resulting in tumorogenesis. Some of malignant cancers and its premalignant lesions were proven to have DSB in their nuclei. The aim of this study was to define the differences in expression of 53BP1 and γ-H2AX, the markers of DSB, among normal, gastric adenoma, and gastric adenocarcinoma tissues.
Methods
Tissue microarray was made with the tissues taken from 121 patients who underwent gastrectomy for gastric adenocarcinoma, and 51 patients who underwent endoscopic mucosal resection for gastric adenoma. Immunochemical stain was performed for the marker of DSB, 53BP1 and γ-H2AX in the tissue microarray. The normal tissues were collected from histologically confirmed tissues with no cellular atypia obtained from the patients with gastric adenocarcinoma.
Results
In gastric carcinoma cells, 53BP1 and γ-H2AX were highly expressed as compared to normal epithelial cells and gastric adenoma (p<0.01). There were no differences in the expression of 53BP1 and γ-H2AX between normal epithelium and gastric adenoma. The expression of 53BP1 in the adenoma with grade II and III atypism was more elevated than in those with grade I atypism. The expression of 53BP1 and γ-H2AX were not significantly different according to the clinicopathologic parameters in the patients with gastric adenocarcinoma.
REFERENCES
1. Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence frequency of eighteen major cancers in 1985. Int J Cancer. 1993; 54:594–606.
2. Lee YC. Gastric adenoma: follow up? Spring conference of Korean Gastroenterol. 2005. 101–108.
3. Lee DS, Kang SB, Baek JT, et al. Immunohistochemical expression of Bcl-2, Bcl-xL, Bax, p53 proteins in gastric adenoma and adenocarcinoma. Korean J Gastroenterol. 2005; 45:394–400.

4. Gorgoulis VG, Vassiliou LV, Karakaidos P, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005; 434:907–913.

5. Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008; 319:1352–1355.

6. Bal N, Yildirim S, Nursal TZ, Bolat F, Kayaselcuk F. Association of ezrin expression in intestinal and diffuse gastric carcinoma with clinicopathological parameters and tumor type. World J Gastroenterol. 2007; 13:3726–3729.

7. Kobayashi J, Iwabuchi K, Miyagawa K, et al. Current topics in DNA double-strand break repair. J Radiat Res. 2008; 49:93–103.

8. Bonner WM, Redon CE, Dickey JS, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008; 8:957–967.
9. Ismail IH, Hendzel MJ. The gamma-H2AX: is it just a surrogate marker of double-strand breaks or much more? Environ Mol Mutagen. 2008; 49:73–82.
10. Kinner A, Wu W, Staudt C, Iliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008; 36:5678–5694.
12. Bartkova J, Horejsí Z, Koed K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005; 434:864–870.

13. Fauci AS, Braunwald E, Kasper DL, et al. Harrison's principles of internal medicine. 17th ed.New York: The McGraw-Hill Companies;2008. 573-574.
Fig. 1.
Visualization of 53BP1 and γ-H2AX expression in normal, adenoma, and gastric adenocarcinoma by immunohistochemistry (×200).
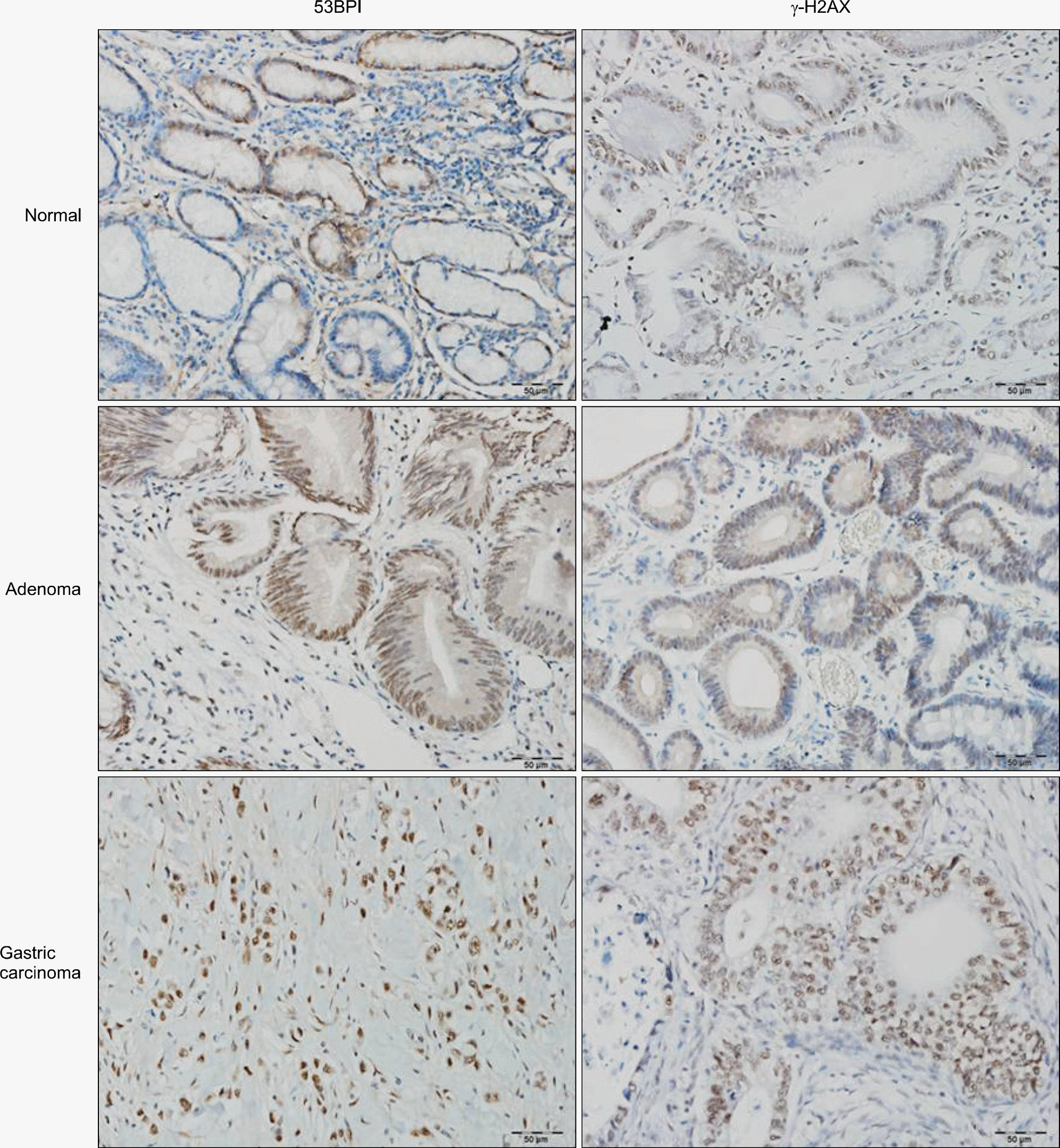
Fig. 2.
Positive immunostaining of protein 53BP1 and γ-H2AX in normal, adenoma and gastric adenocarcinoma. ∗ Significantly higher compared to normal tissues (p=0.000 in 53BP1, p=0.016 in γ-H2AX).
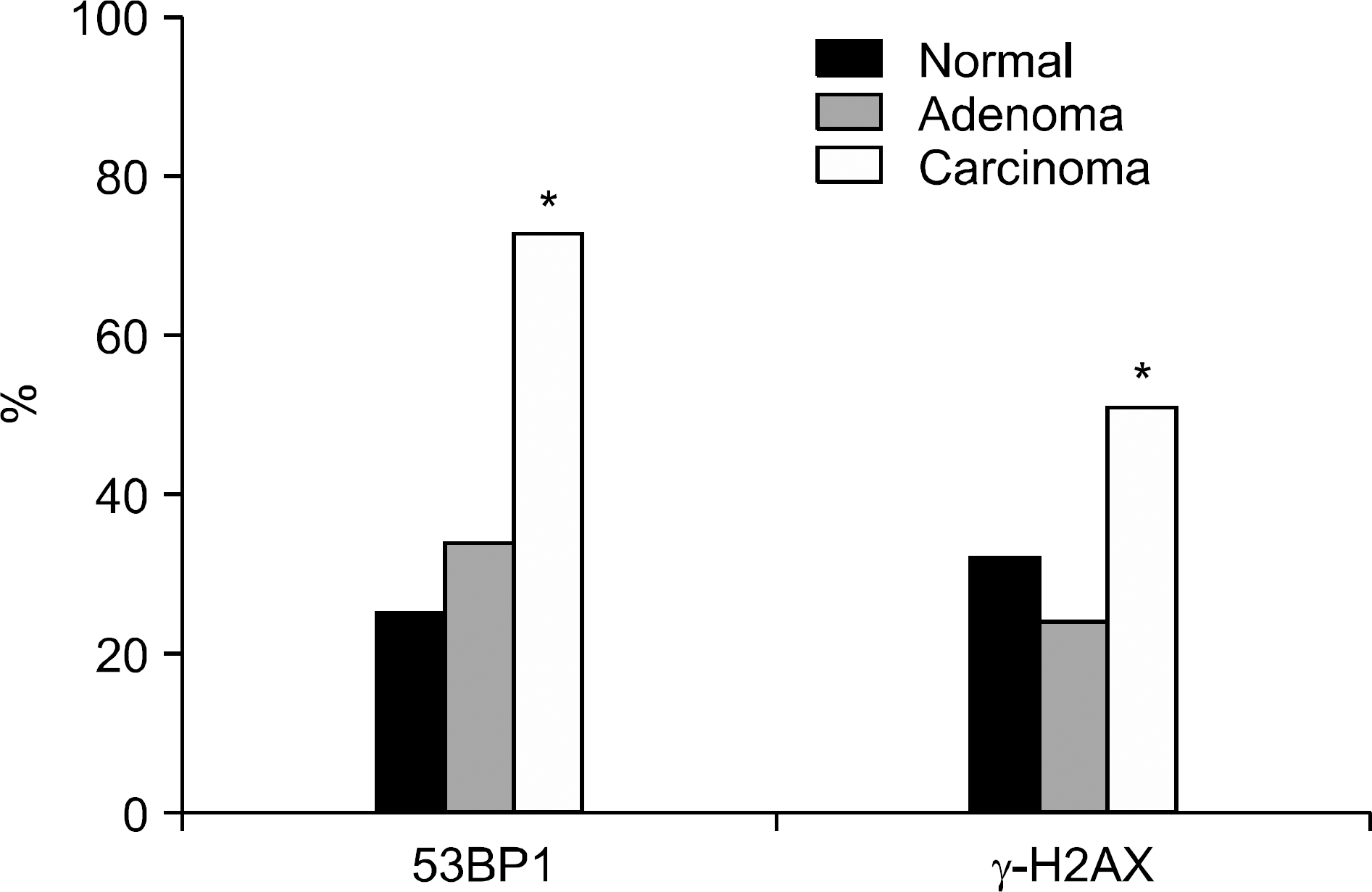
Table 1.
Mean Expression Score of 53BP1 and γ-H2AX in Normal, Adenoma, and Gastric Adenocarcinoma
| 53BP1 | γ-H2AX | |
|---|---|---|
| Normal (n=121) | 0.57±1.15 | 0.88±1.41 |
| Adenoma (n=51) | 0.97±1.47 | 0.69±1.39 |
| Gastric adenocarcinoma (n=121) | 3.36±2.91∗† | 2.32±3.47∗† |
Table 2.
Mean Expression Score of 53BP1 and γ-H2AX in Gastric Adenoma according to the Atypism
| Atypism | 53BP1 | γ-H2AX |
|---|---|---|
| I (n=20) | 0.08±0.29 | 1.10±1.79 |
| II (n=16) | 1.57±1.65 | 0.57±1.28 |
| III (n=15) | 1.22±1.64 | 0.20±0.45 |
| p value | 0.045 | NS |
Table 3.
Summary of Mean Expression Score of 53BP1 and γ-H2AX in Gastric Adenocarcinoma according to the Clinicopathologic Parameters




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download