Abstract
Background/Aims
Endoscopic submucosal dissection (ESD) is commonly used for radical resection of gastric adenoma and mucosal cancer, but there is about 30% of discrepancy rate between the histology of the endoscopic biopsy and that of the resected specimen obtained from the same lesion by ESD. The aim of this study was to clarify the clinical significance of IL-6, VEGF, CRP before ESD.
Methods
We investigated the correlation between serum IL-6, VEGF, CRP level and discrepancy rate of gastric neoplastic lesions (10 low-grade dysplasias, 18 high-grade dysplasias, and 25 early gastic cancers).
Results
Serum levels of IL-6 in gastric adenoma and mucosal cancer patients were significantly higher than in healthy controls (p<0.05). Especially, serum IL-6 level of high-grade dysplasia patient was significantly higher than low-grade dysplasia and mucosal cancer patients, and the positive rate, sensitivity, and negative predictive value of serum IL-6 levels were higher in high-grade dysplasia patient compared to low-grade dysplasia patient and mucosal cancer patient. Serum levels of VEGF in patients with gastric adenoma and mucosal cancer were significantly higher than healthy controls (p<0.01). Serum levels of CRP in patients with mucosal cancer were significantly higher than in the controls (p<0.05), and the positive rate, sensitivity, and positive predictive value of serum CRP levels were higher in high-grade dysplasia and mucosal cancer patients compared to low-grade dysplasia patient.
REFERENCES
1. Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986; 8:1–27.

2. Zinvy J, Wang TC, Yantiss R, et al. Role of therapy or monitoring in preventing progression to gastric cancer. J Clin Gastroenterol. 2003; 36:50–60.
3. Ohno S, Fujii T, Ueda S, et al. Predictive factors and timing for liver recurrence after curative resection of gastric carcinoma. Am J Surg. 2003; 185:258–263.

4. Cahill RJ, Kilgallen C, Beattie S, et al. Gastric epithelial cell kinetics in the progression from normal mucosa to gastric carcinoma. Gut. 1996; 38:177–181.

5. Bodger K, Crabtree JE. Helicobacter pylori and gastric inflammation. Br Med Bull. 1998; 54:139–150.
6. Blaser MJ, Parsonnet J. Parasitism by the ‘slow’ bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest. 1994; 94:4–8.
7. Forman D. Helicobacter pylori infection and cancer. Br Med Bull. 1998; 54:71–78.
8. O'Connor F, Buckley M, O'Morain C. Helicobacter pylori: the cancer link. J R Soc Med. 1996; 88:674–678.
9. Chatelain D, de Lajarte Thirouard AS, Tiret E, Flejou JF. Adenocarcinoma of the upper esophagus arising in heterotopic gastric mucosa: common pathogenesis with Barrett's adenocarcinoma? Virchows Arch. 2002; 441:406–411.

10. Tanahashi T, Kita M, Kodama T, et al. Cytokine expression and production by purified Helicobacter pylori urease in human gastric epithelial cells. Infect Immun. 2000; 68:664–671.
11. Yamaoka Y, Kodama T, Kita M, et al. Relation between cy-tokines and Helicobacter pylori in gastric cancer. Helicobacter. 2001; 6:116–124.
12. Arii K, Tanimura H, Iwahashi M, et al. Neutrophil functions and cytokine production in patients with gastric cancer. Hepatogastroenterology. 2000; 47:291–297.
13. Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991; 325:1127–1131.
14. Senger DR, Brown LF, Claffey KP, et al. Vascular permeability factor, tumor angiogenesis and stroma generation. Invasion Metastasis. 1994; 14:385–394.
15. Ikeguchi M, Oka S, Saito H, et al. The expression of vascular endothelial growth factor and proliferative activity of cancer cells in gastric cancer. Langenbeck's Arch Surg. 1999; 384:264–270.

16. Maehara Y, Kabashima A, Koga T, et al. Vascular invasion and potential for tumor angiogenesis and metastasis in gastric carcinoma. Surgery. 2000; 128:408–416.

17. Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996; 271:736–741.

18. Kallio R, Surcel HM, Bloigu A, et al. C-reactive protein, pro-calcitonin and interleukin-8 in the primary diagnosis of infections in cancer patients. Eur J Cancer. 2000; 36:889–894.

19. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. Gastric Cancer. 1998; 1:10–24.
20. Kim HK, Song KS, Park YS, et al. Elevated levels of circulating platelet microparticles, VEGF, IL6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003; 39:184–191.

21. Jang JS, Lee EJ, Lee SW, et al. Endoscopic submucosal dissection for early gastric cancer and gastric adenoma. Korean J Gastroenterol. 2007; 49:256–263.
22. Min BH, Lee JH, Kim JJ, et al. Clinical outcome of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopicy mucosal resection after circumferential precutting (EMR-P). Dig Liver Dis. 2009; 41:201–209.
23. Park EH, Choi SR, Jeong JS, et al. The histologic discrepancy before and after endoscopic submucosal dissection of gastric adenoma and early gastric cancer. Korean J Gastrointest Endosc. 2007; 34:125–131.
24. Yamada H, Ikegami M, Shimoda T, et al. Longterm follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004; 36:390–396.

25. Hay LJ. Surgical management of gastric polyp and adenomas. Surgery. 1956; 39:114–119.
26. Jo YJ, Lee SJ, Jo JY, et al. The effectiveness of biomarkers to detect micrometastasis of gastric adenocarcinoma. J Gastroenterol Hepatol. 2005; 20:45.
27. Oh SY, Kwon HC, Kim SY, et al. Clinicopathologic significance of HIF-1α, p53, and VEGF expression and preoperative serum VEGF level in gastric cancer. BMC Cancer. 2008; 8:123.

28. Rugge M, Cassaro M, Di Mario F, et al. The long term outcome of gastric non-invasive neoplasia. Gut. 2003; 52:111–116.

29. Wu CW, Wang SR, Chao MF, et al. Serum interleukin-6 levels reflect disease status of gastric cancer. Am J Gastroenterol. 1996; 91:1417–1422.
30. Yonemura Y, Endo Y, Fujita H, et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res. 1999; 5:1823–1829.
31. Huang SP, Wu MS, Wang HP, et al. Correlation between serum levels of interleukin-6 and vascular endothelial growth factor in gastric carcinoma. J Gastroenterol Hepatol. 2002; 17:1165–1169.

Fig. 1.
Pathologic findings after endoscopic sbumucosal dissection: low-grade dysplasia (A), high-grade dysplasia (B), and mucosal cancer (C) (H&E stain, ×400).
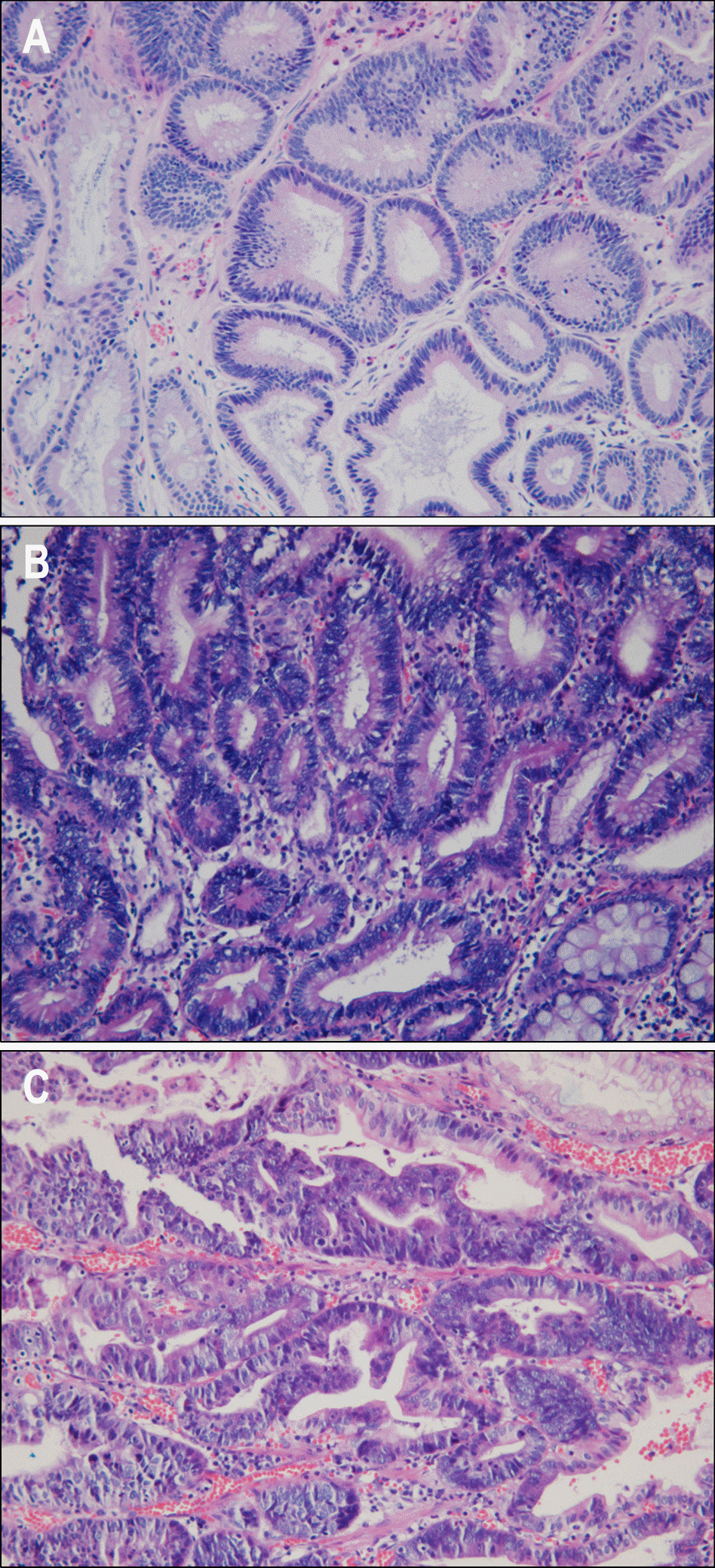
Fig. 2.
Serum levels of IL-6 in controls, gastric adenoma and mucosal cancer patients. LGD, low-grade dysplasia; HGD, high-grade dysplasia; EGC, early gastric cancer.
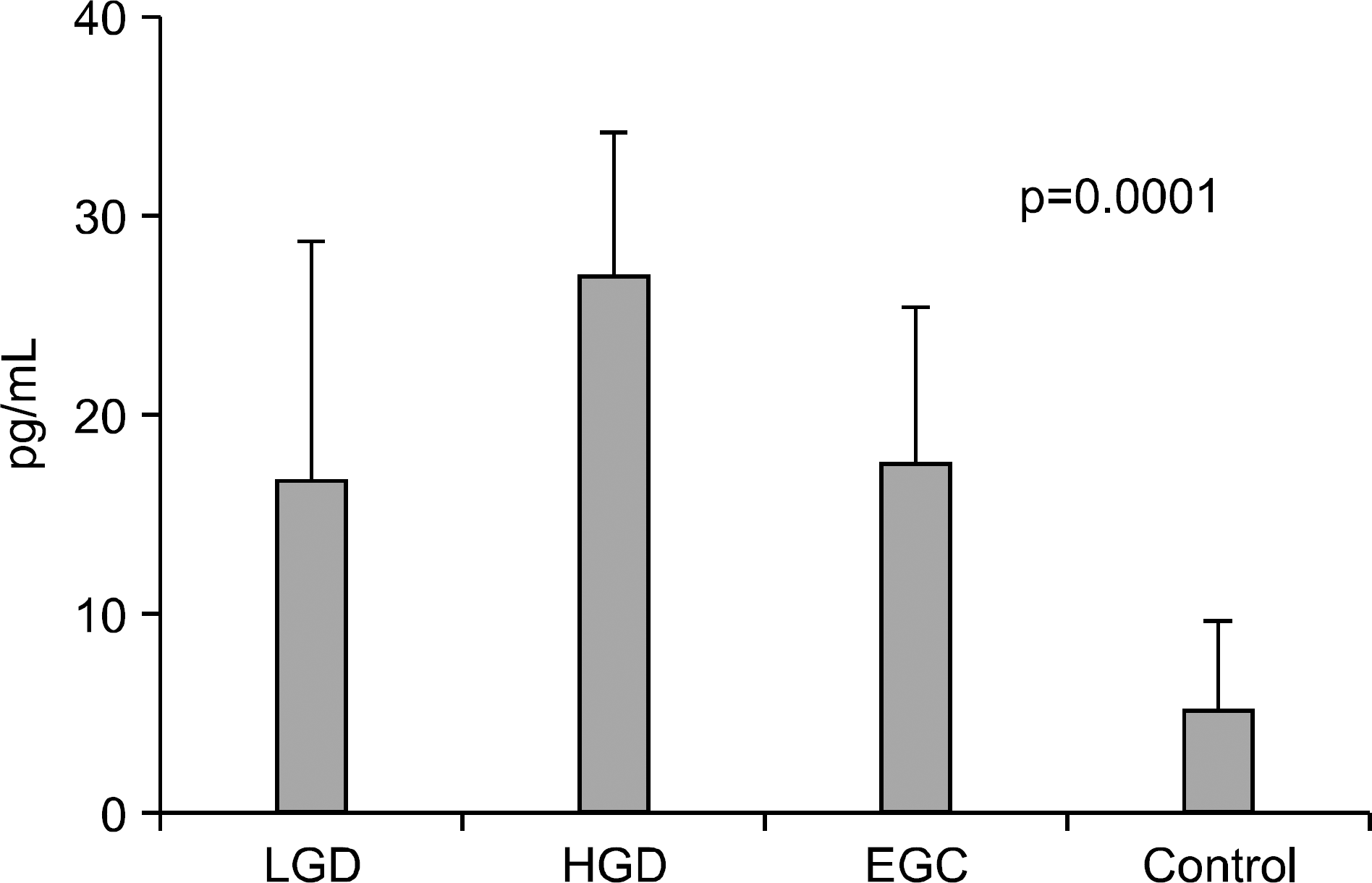
Fig. 3.
Serum levels of VEGF in controls, gastric adenoma and mucosal cancer patients. LGD, low-grade dysplasia; HGD, high-grade dysplasia; EGC, early gastric cancer.
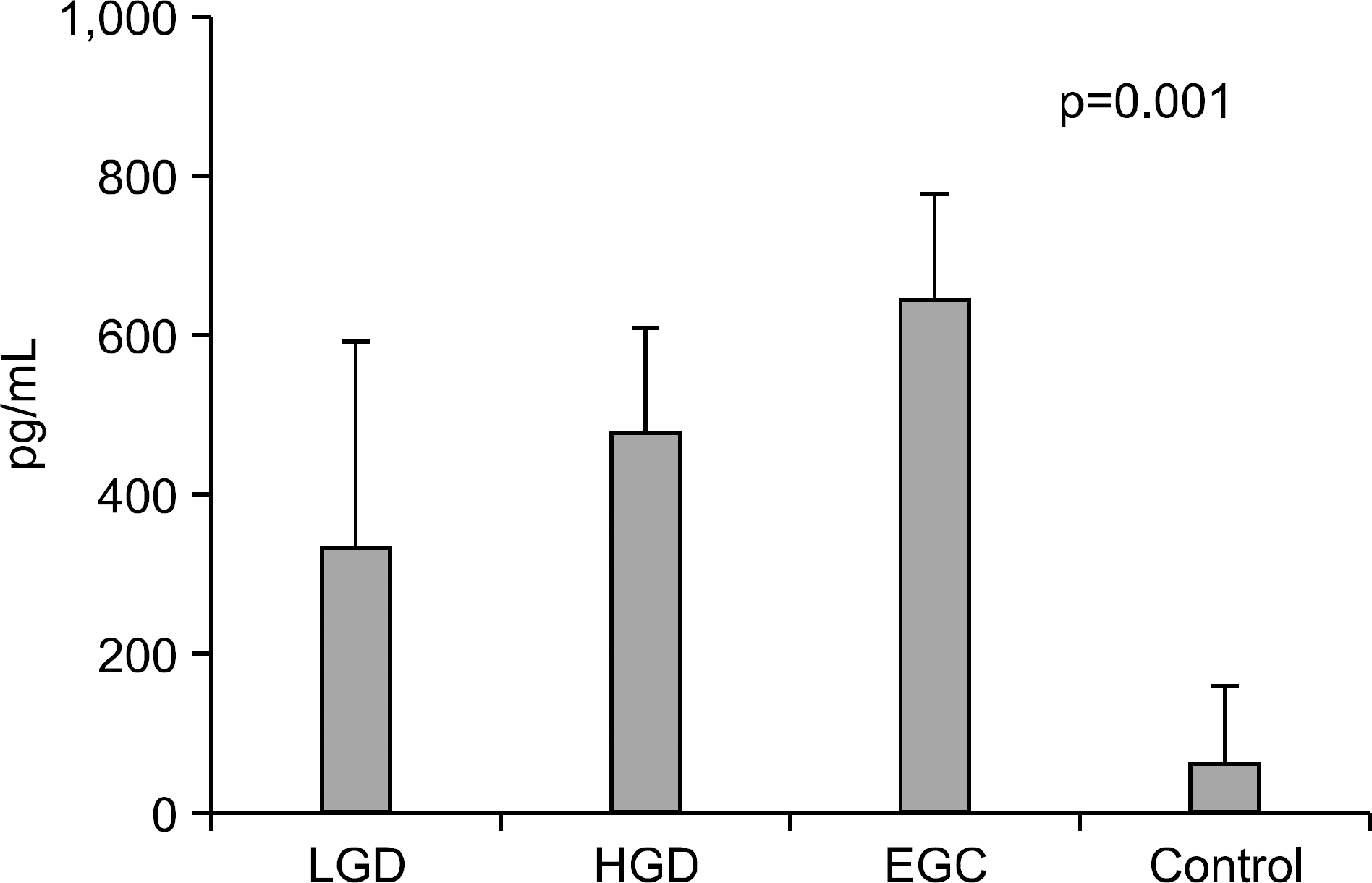
Fig. 4.
Serum levels of CRP in controls, gastric adenoma, and mucosal cancer patients. LGD, low-grade dysplasia; HGD, high-grade dysplasia; EGC, early gastric cancer.
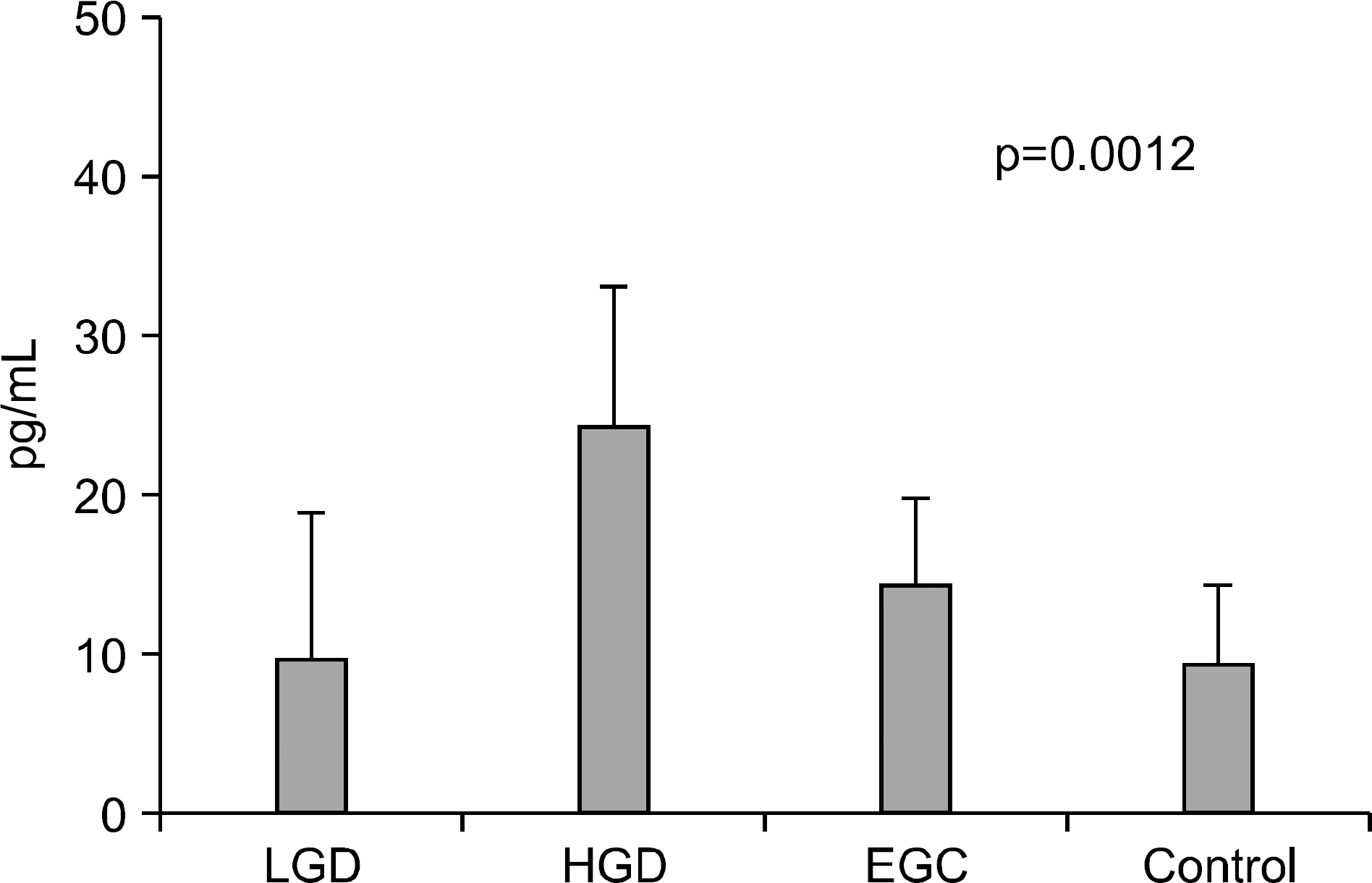
Table 1.
Dermographic Data of Patients
| LGD | HGD | M cancer | Control | |
|---|---|---|---|---|
| No. of patients | 10 | 18 | 25 | 60 |
| Age | ||||
| Median (range) | 61.5 | 61.7 | 59.6 | 58.1 |
| (40-68) | (42-69) | (37-76) | (34-75) | |
| Sex | ||||
| Male | 6 | 10 | 15 | 37 |
| Female | 4 | 8 | 10 | 23 |
Table 2.
Serum Levels of IL-6, VEGF, CRP in the Controls, Adenoma, Cancer Groups
Table 3.
Serum Levels of IL-6 in the Controls, Adenoma, Cancer Groups according to Age
Table 4.
Positive Rate of Serum IL-6, VEGF, CRP in Gastric Adenoma Patients and Cancer Patients
Table 5.
The Sensitivity and Specificity of Three Parameters in Patients with Gastric Adenoma and Cancer Patients
| IL-6 | VEGF | CRP | |
|---|---|---|---|
| Sensitivity (%) | |||
| LGD | 60.0 | 100 | 10.0 |
| HGD | 77.8 | 100 | 77.8 |
| M cancer | 44.0 | 100 | 60.0 |
| Specificity (%) | |||
| LGD | 20.0 | 63.3 | 60.0 |
| HGD | 20.0 | 63.3 | 60.0 |
| M cancer | 20.0 | 63.3 | 60.0 |
Table 6.
The Positive Predictive Value and Negative Predictive Value of Serum IL-6, VEGF, CRP in Gastric Adenoma and Cancer Patients
| LGD | HGD | M cancer | ||||
|---|---|---|---|---|---|---|
| PPV (%) | NPV (%) | PPV (%) | NPV (%) | PPV (%) | NPV (%) | |
| IL-6 | 11.00 | 75.00 | 22.58 | 75.00 | 18.33 | 50.00 |
| VEGF | 31.25 | 100 | 45.00 | 100 | 53.19 | 100 |
| CRP | 4.00 | 80.00 | 36.84∗ | 90.00 | 38.46∗ | 78.26 |
Table 7.
The Probability of Serum IL-6, VEGF, CRP in Gastric Adenoma and Cancer Patients
| Probability (%) | |||
|---|---|---|---|
| LGD | HGD | M cancer | |
| IL-6 | 25.71 | 33.33 | 12.94 |
| VEGF | 68.57 | 71.80 | 74.12 |
| CRP | 60.00 | 73.53 | 60.00 |
Table 8.
Serum Levels of VEGF in the Controls, Adenoma Cancer Groups according to Age
Table 9.
Serum Levels of CRP in Controls, Adenoma, Cancer Groups According to Age




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download