This article has been corrected. See "Erratum: Correction of Title. Correlation Between Lower Urinary Tract Symptoms and Premature Ejaculation in Korean Men Older Than 40 Years Old" in Volume 55 on page 434.
Abstract
Purpose
To evaluate the correlation between lower urinary tract symptoms (LUTS) and premature ejaculation (PE) in Korean men older than 40 years.
Materials and Methods
In total, 258 men older than 40 years completed the International Prostate Symptom Score (IPSS; total score, storage symptoms [ST], and voiding symptoms [VD]), a 5-item version of the International Index of Erectile Function (IIEF-5), and the Premature Ejaculation Diagnostic Tool (PEDT). The study examined the relationship between LUTS and PE. In the PEDT, PE is defined as a score ≥11.
Results
The prevalence of PE was 29.1% with the PEDT versus a self-reported value of 49.5%. The prevalence of PE was 30.9% in 40 to 59-year-old men (21.3%) and 28.1% in 60 to 79 year-old men (78.7%). In men 40 to 59 and 60 to 79 years old, the mean PEDT, IPSS, and IIEF-5 scores were 8.65 and 7.88, 13.5 and 12.38, and 15.83 and 13.69, respectively. No significant correlations were observed between the total and subscale scores of the IPSS (p=0.204) and the PEDT (p=0.309) with increasing age, whereas a significant negative correlation was detected between the IIEF-5 and age (p=0.002). The PEDT score was significantly correlated with the IPSS-ST (r=0.326, p<0.001), IPSS-VD (r=0.183, p=0.005), IPSS-total (r=0.310, p<0.001), and IIEF-5 total (r=-0.248, p<0.001).
Worldwide, the human life span and the proportion of elderly people in the population have both increased. Various changes occur in the human body with age. In elderly men, lower urinary tract symptoms (LUTS) and sexual dysfunction often appear concurrently. Several community-based studies have shown strong correlations between the prevalence of sexual dysfunction, especially erectile dysfunction (ED), and the severity of LUTS with increasing age. This coexistence of sexual problems with LUTS negatively affects the quality of life (QoL) [1]. The prevalence of LUTS and sexual dysfunction increases with age in Korea, and both present synchronously in many cases. Consequently, effort has been directed at determining the pathophysiology common to these conditions, including hyperactive adrenergic signaling, increased Rho-kinase and endothelium activity, and decreased nitric oxide (NO) levels in genital tissue.
Premature ejaculation (PE) is also seen in the elderly as a primary or secondary condition. In many countries, the prevalence of PE in most decades is similar, about 20 to 30% [2]. By contrast, in Korea, the prevalence of PE in the sixth decade is 36.8% vs. 24.6% in the third decade [3]. The reason for the increased prevalence of PE with age has not been determined, but it could be related to conditions such as LUTS and ED.
If the increased prevalence of PE during old age in Korea is the result of increasing secondary PE compared to primary PE [3], then a correlation may exist between ED or LUTS in middle-aged to elderly men with PE. Therefore, this study examined the correlations among LUTS, ED, and PE in Korean men older than 40 years.
This study enrolled 258 men older than 40 years presenting between June 2010 and March 2011 in Busan, Korea. All subjects were from the general population and had participated in a health care lecture in Busan. Men between 40 and 79 years old who were willing and able to participate in the study were included. Subjects who were younger than 40 years old or older than 79 years old were excluded. The study had a cross-sectional design.
All subjects were assessed by using the International Prostate Symptom Score (IPSS), a 5-item version of the International Index of Erectile Function (IIEF-5) from the ED domain of the IIEF [4], and a validated Korean version of the Premature Ejaculation Diagnostic Tool (PEDT) [5]. A PEDT score ≥11 was categorized as PE. The IPSS was categorized into the total score, storage symptoms (ST), and voiding symptoms (VD). The relationships among the storage symptoms, voiding symptoms, IIEF-5 scores, and PEDT scores were analyzed.
The statistical analyses were performed by using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Student's t-test was used to examine the differences in the IPSS-ST, IPSS-VD, IPSS-total, IIEF-5, and PEDT between the age groups. Spearman's rank correlation analysis was used to examine the correlations of the IPSS and IIEF-5 with the PEDT and each other. The correlation analyses were stratified by patient age. The results were considered statistically significant when p<0.05.
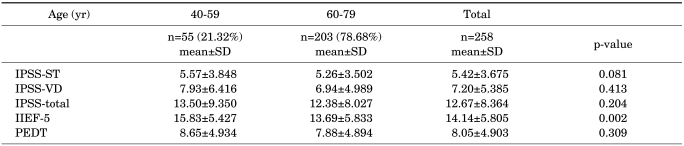
The study enrolled 258 men: 55 (21.32%) were 40 to 59 years old and 203 (78.68%) were 60 to 79 years old. The mean IPSS, IIEF, and PEDT were 12.67, 14.14, and 8.05, respectively. Using the PEDT, the prevalence of PE was 29.1% versus a self-reported incidence of 49.5%.
Comparing the age groups (40 to 59 vs. 60 to 79 years old), no significant correlations were observed in the total and subscale scores of the IPSS and PEDT (IPSS-ST, p=0.081; IPSS-VD, p=0.413; IPSS-total, p=0.204; PEDT, p=0.309). However, a significant negative correlation was detected between the IIEF score and age (p=0.002). The mean±SD IIEF-5 score was 15.83±5.427 in those aged 40 to 59 years old and 13.69±5.833 in those aged 60 to 79 years old (Table 1).
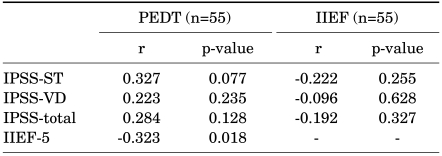
Comparing the assessment scales in the 40 to 59 year-old age group, the PEDT score was significantly correlated with IIEF (r=-0.323, p=0.018), but was not correlated with IPSS-ST (r=0.327, p=0.077), IPSS-VD (r=0.223, p=0.235), or IPSS-total (r=0.284, p=0.128). The IIEF-5 score was not correlated with IPSS-ST (r=-0.222, p=0.255), IPSS-VD (r=-0.096, p=0.628), or IPSS-total (r=-0.192, p=0.327) (Table 2).
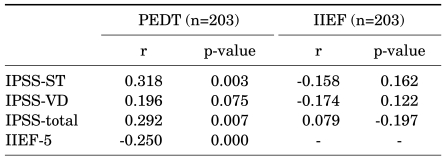
Comparing the assessment scales in the 60 to 79 year-old age group, the PEDT score was significantly correlated with the IPSS-ST (r=0.318, p=0.003), IPSS-total (r=0.292, p=0.007), and IIEF-5 (r=-0.250, p<0.001), but was not correlated with the IPSS-VD (r=0.196, p=0.075). The IIEF-5 score was significantly negatively correlated with the PEDT (r=-0.250, p<0.001), but was not correlated with the IPSS-ST (r=-0.158, p=0.162), IPSS-VD (r=-0.174, p=0.122), or IPSS-total (r=-0.197, p=0.079) (Table 3).
Comparing the domains of the IIEF-5 with each domain of the PEDT, questions 1, 4, and 5 of the PEDT were significantly negatively correlated with all domains of the IIEF-5. The frequencies of the PE and ejaculation with minimal sexual stimulation domains were significantly correlated with the intercourse satisfaction domain of the IIEF-5.
Ejaculation is triggered by efferent dopamine acting on the D2 receptors of central and spinal efferent fibers, which relay information down to the sympathetic ganglia at T10-L2 and the sacral fibers [6]. This stimulates pudendal nerve fibers from the S2-S4 region of the spinal cord, resulting in smooth muscle contractions of the prostate, seminal vesicles, vas deferens, and epididymis. This increases the volume and fluid content of semen, which is forced into the posterior urethra under the control of the sympathetic nervous system, producing emission [7].
The Diagnostic and Statistical Manual of Mental Disorders, 4 edition, test revision (DSM-IV-TR) defines PE as "the persistence of recurrent ejaculation with minimal sexual stimulation before, on, or shortly after penetration and before the person wishes it and it causes marked distress or interpersonal difficulty and is not due to the direct effects of a substance" [8].
Primary PE (lifelong) occurs and persists from the first sexual encounter, whereas secondary PE (acquired) transpires after a period of normal control of ejaculatory function. Primary PE likely has a genetic basis. One study reported that 91% of men with primary PE had a first-degree relative with PE [9]. The approach to treating secondary or acquired PE is to treat the underlying condition. Secondary PE is due to psychological or relationship problems, ED, prostatitis, or thyroid dysfunction [10]. The most common cause of secondary PE is declining erectile function, whereby rapid ejaculation becomes a compensatory mechanism, either consciously or unconsciously, for the inability to maintain the erection. An alternative explanation is that lower levels of NO and increased sympathetic tone, associated with aging, both predispose men to erectile failure and hasten ejaculation [11-13].
The PEDT has been found to have a high level of agreement with clinical diagnosis, and its test-retest reliability has been shown to be good, with an intra-class correlation coefficient of 0.88 [14]. The PEDT consists of five questions that address the following five domains: ejaculation control, frequency of PE, ejaculation with minimal sexual stimulation, distress, and interpersonal difficulty. Each question has five responses, and the scores of each question range from 0 to 4 with a minimum total score of 0 and a maximum score of 20. A low score suggests a low probability of having PE.
In the Premature Ejaculation Perceptions and Attitudes study, many countries did not show an increased prevalence of PE with increasing age [15]. However, according to the data of the Korean Society for Sexual Medicine and Andrology, Koreans demonstrate an increased PE prevalence with increasing age; these data support the above hypothesis and concur with our data.
Our study confirmed that PE was correlated with ED. Furthermore, ejaculation with very little stimulation and the frequency of PE were not correlated with ED. Nevertheless, difficulty with ejaculation control was correlated with ED. In particular, erection firmness and erection maintenance ability were correlated with the control of ejaculation, as seen in secondary PE.
The proposal has been made that the use of a PDE-5 inhibitor (e.g., sildenafil) may increase the level of NO centrally (reducing sympathetic drive) and peripherally (leading to smooth muscle dilatation of the vas deferens and seminal vesicles, opposing sympathetic vasoconstriction), probably prolonging the intravaginal ejaculation latency time (IELT) in men with PE [16]. Nitric oxide synthase isoenzymes are present in human seminal vesicle smooth muscle, and the NO-cGMP and NO-cAMP signaling pathways are not only involved in the relaxation of human penile smooth muscles but also affect smooth muscle relaxation in the vas deferens, seminal vesicle, prostate, urethra, and skeletal muscles [17].
In a randomized placebo-controlled study of men with primary PE without ED, sildenafil did not significantly prolong the IELT, but it was associated with a perception of greater control over ejaculation in men receiving active therapy. Our cross-sectional study showed that an improvement in ED was correlated with an improvement in PE. However, we did not examine the correlation between the use of PDE-5 inhibitors and PE.
Several community-based studies have shown strong correlations between the prevalence of sexual dysfunction, especially ED, and the severity of LUTS with increasing age [1]. In our study, the IPSS-ST, IPSS-VD, IPSS-total, and PEDT scores were not significantly correlated with age. Only the IIEF-5 score had a significant correlation with age.
The pathophysiological mechanism of the correlations among LUTS, ED, and PE remains unclear, although several hypotheses have been proposed [18-21]. One possible cause is overactivation of the autonomic nervous system and increased sympathetic nervous system activity. According to this hypothesis, LUTS could result from sympathetic nervous system tone, which induces the occurrence of urinary storage symptoms owing to the contraction of smooth muscles in the prostate gland and urinary bladder. ED occurs as a result of smooth muscle contraction in the corpus cavernosum. PE occurs as a result of smooth muscle contraction in the prostate, seminal vesicle, vas deferens, and epididymis [18-21].
However, this hypothesis has not been confirmed in experimental studies. In our study, the PEDT score was significantly correlated with the IPSS-ST, IPSS-total, and IIEF-5 scores in the 60 to 79 year-old age group. The PEDT score was significantly correlated with IPSS-ST; however, it was not correlated with IPSS-VD. PE seems to correlate with LUTS and erectile dysfunction, and storage symptoms seemed to be correlated with PE more than voiding symptoms in the 60 to 79 year-old men in our study. This is explained by the increased sympathetic tone in old age. Some LUTS result from increased sympathetic tone and storage symptoms, which were related more to the PEDT score, and are associated with increased sympathetic tone rather than voiding symptoms.
Irwin et al. [22] reported that an overactive bladder was significantly associated with an increased prevalence of ED and that sexual activity, sexual enjoyment, and sexual satisfaction were reduced as the result of urinary symptoms. In our study, however, the IIEF-5 score had the only significant correlation with the PEDT score. The IIEF-5 score was not significantly correlated with the IPSS-ST, IPSS-VD, or IPSS-total. In fact, our previous study revealed that the IIEF-5 score was significantly negatively correlated with the IPSS-total, especially IPSS-ST [23]. The reason for this difference can be explained by the fact that the population of the previous study consisted of patients with LUTS, whereas the population in the current study was a general population who did not visit the hospital at the time the questionnaire was administered. Therefore, a smaller proportion of the population of this study complained of LUTS.
In recent years, many studies have reported that the administration of alpha-blockers improves LUTS and sexual dysfunction [24,25]. Other studies have reported that not only the bladder neck, urethra, and prostate, but also the seminal vesicle and vas deferens show a typical distribution for alpha-adrenoceptors, which accounts for their capacity to affect ejaculation, as reported in clinical studies in men with prostatic hyperplasia (up to 7% of cases reported with tamsulosin) [26]. Almost no reports have addressed the effects of antimuscarinic agents on sexual function in LUTS. Our study showed that PE was significantly correlated with LUTS, particularly storage symptoms, in the 60 to 79 year-old age group rather than the 50 to 69 year-old age group. In our study, no information on the patients' medical history was collected. To address this limitation, a well-designed, larger-scale assessment of the relationship and treatment of LUTS, ED, and PE is warranted to validate the results of this study.
Korean middle-aged to elderly men frequently experience PE, which is significantly correlated with ED. In addition, LUTS, particularly storage symptoms, have a negative effect on PE. An improvement in storage symptoms could positively affect men with PE and ED, and the storage symptoms of LUTS may play an important role in the treatment of PE in middle-aged to elderly Korean men.
References
1. Rosen RC, Wei JT, Althof SE, Seftel AD, Miner M, Perelman MA, et al. Association of sexual dysfunction with lower urinary tract symptoms of BPH and BPH medical therapies: results from the BPH Registry. Urology. 2009; 73:562–566. PMID: 19167031.

2. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999; 281:537–544. PMID: 10022110.
3. Park HJ, Park JK, Park K, Lee SW, Kim SW, Yang DY, et al. Prevalence of premature ejaculation in young and middle-aged men in Korea: a multicenter internet-based survey from the Korean Andrological Society. Asian J Androl. 2010; 12:880–889. PMID: 20676115.

4. Chung TG, Lee TK, Chung S, Lee MS, Kim YS, Ahn TY. The Korean version of the International Index of Erectile Function (IIEF): reliability and validation study. Korean J Urol. 1999; 40:1334–1343.
5. Kam SC, Han DH, Huh JH, Lee SW. Development and validation of a Korean version of the premature ejaculation diagnostic tool (PEDT). Korean J Androl. 2009; 27:185–193.
6. Kimura Y, Miyamoto A, Urano S, Tadano T, Kisara K. The spinal monoaminergic systems relating to ejaculation. I. Ejaculation and dopamine. Andrologia. 1982; 14:341–346. PMID: 6181718.

7. Palmer NR, Stuckey BG. Premature ejaculation: a clinical update. Med J Aust. 2008; 188:662–666. PMID: 18513177.

8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text revision: DSM-IV-TR. 2000. 4th ed. Washington, DC: American Psychiatric Association.
9. Waldinger MD, Rietschel M, Nöthen MM, Hengeveld MW, Olivier B. Familial occurrence of primary premature ejaculation. Psychiatr Genet. 1998; 8:37–40. PMID: 9564687.

10. Jannini EA, Lombardo F, Lenzi A. Correlation between ejaculatory and erectile dysfunction. Int J Androl. 2005; 28(Suppl 2):40–45. PMID: 16236063.

11. Waldinger MD. Premature ejaculation: state of the art. Urol Clin North Am. 2007; 34:591–599. vii–viii. PMID: 17983899.

12. Earle CM, Stuckey BG, Ching HL, Wisniewski ZS. The incidence and management of priapism in western Australia: a 16 year audit. Int J Impot Res. 2003; 15:272–276. PMID: 12934055.

13. Chew KK, Stuckey BG, Earle CM, Dhaliwal SS, Keogh EJ. Penile fibrosis in intracavernosal prostaglandin E1 injection therapy for erectile dysfunction. Int J Impot Res. 1997; 9:225–229. PMID: 9442421.

14. Symonds T, Perelman M, Althof S, Giuliano F, Martin M, Abraham L, et al. Further evidence of the reliability and validity of the premature ejaculation diagnostic tool. Int J Impot Res. 2007; 19:521–525. PMID: 17568761.

15. Porst H, Montorsi F, Rosen RC, Gaynor L, Grupe S, Alexander J. The premature ejaculation prevalence and attitudes (PEPA) survey: prevalence, comorbidities, and professional help-seeking. Eur Urol. 2007; 51:816–823. PMID: 16934919.

16. McMahon CG, Stuckey BG, Andersen M, Purvis K, Koppiker N, Haughie S, et al. Efficacy of sildenafil citrate (Viagra) in men with premature ejaculation. J Sex Med. 2005; 2:368–375. PMID: 16422868.

17. Dixon JS, Jen PY. Development of nerves containing nitric oxide synthase in the human male urogenital organs. Br J Urol. 1995; 76:719–725. PMID: 8535715.

18. Taylor JM, Desouza R, Wang R. Common approach to managing lower urinary tract symptoms and erectile dysfunction. Asian J Androl. 2008; 10:45–53. PMID: 18087643.

19. McVary KT, McKenna KE. The relationship between erectile dysfunction and lower urinary tract symptoms: epidemiological, clinical, and basic science evidence. Curr Urol Rep. 2004; 5:251–257. PMID: 15260924.

20. McVary KT. Erectile dysfunction and lower urinary tract symptoms secondary to BPH. Eur Urol. 2005; 47:838–845. PMID: 15925081.

21. Rosen RC, Giuliano F, Carson CC. Sexual dysfunction and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH). Eur Urol. 2005; 47:824–837. PMID: 15925080.

22. Irwin DE, Milsom I, Reilly K, Hunskaar S, Kopp Z, Herschorn S, et al. Overactive bladder is associated with erectile dysfunction and reduced sexual quality of life in men. J Sex Med. 2008; 5:2904–2910. PMID: 19090944.

23. Oh SY, Min KS, Choi SH. Effect of prostate volume and lower urinary tract symptoms on erectile function. Korean J Urol. 2007; 48:24–28.
24. Chung BH, Lee JY, Lee SH, Yoo SJ, Lee SW, Oh CY. Safety and efficacy of the simultaneous administration of udenafil and an alpha-blocker in men with erectile dysfunction concomitant with BPH/LUTS. Int J Impot Res. 2009; 21:122–128. PMID: 19194451.
25. Nickel JC, Elhilali M, Emberton M, Vallancien G. Alf-One Study Group. The beneficial effect of alfuzosin 10 mg once daily in 'real-life' practice on lower urinary tract symptoms (LUTS), quality of life and sexual dysfunction in men with LUTS and painful ejaculation. BJU Int. 2006; 97:1242–1246. PMID: 16686719.

26. Moriyama N, Nasu K, Takeuchi T, Akiyama K, Murata S, Nishimatsu H, et al. Quantification and distribution of alpha 1-adrenoceptor subtype mRNAs in human vas deferens: comparison with those of epididymal and pelvic portions. Br J Pharmacol. 1997; 122:1009–1014. PMID: 9401762.
FIG. 1
Significant correlation between Premature Ejaculation Diagnostic Tool score and International Prostate Symptom Score (IPSS)-storage symptoms domain in men aged 60 to 79 years (n=203).
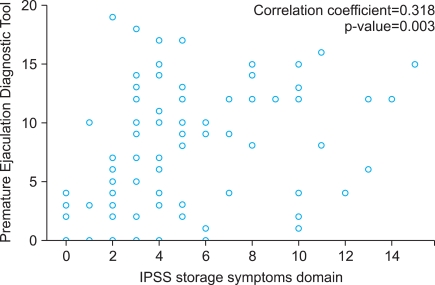
TABLE 4
Correlation of each domain of the IIEF-5 with each domain of the PEDT
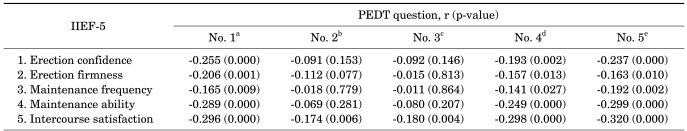
IIEF, International Index of Erectile Function; PEDT, Premature Ejaculation Diagnostic Tool; r, correlation coefficient.
a: How difficult is it for you to delay ejaculation (ejaculation control)?, b: Do you ejaculate before you want to (frequency of premature ejaculation)?, c: Do you ejaculate with very little stimulation (ejaculation with minimal sexual stimulation)?, d: Do you feel frustrated because you ejaculate before you want to (distress)?, e: How concerned are you that your time to ejaculation leaves your partner sexually unfulfilled (interpersonal difficulty)?.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download