Mesenteric fibromatosis (MF) is a rare, benign, intra-abdominal tumor. MF is characterized by proliferating fibrous tissue in the bowel mesentery. Although MF occasionally invades the bowel or adjacent tissues with aggressive myofibroblastic proliferation, MF lacks the capacity of malignant tumorigenesis with distant metastasis [1]. MF is frequently associated with Gardner's syndrome, previous trauma, prolonged estrogen intake, and pregnancy, but MF can occur as a primary condition in the absence of predisposing factors [2]. MF-induced ureteral stenosis is a very rare urological problem. We present a case of primary MF causing ureteral stenosis with a review of the relevant literature.
CASE REPORT
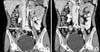
A 46-year-old woman presented with intermittent right flank pain she had experienced for a year. The patient had previously been healthy and had no history of disease. Physical examination revealed no significant findings such as tenderness at the costovertebral angle area, a palpable mass, or peripheral lymphadenopathy. Urinalysis, a complete blood count, and routine blood biochemistry tests showed no abnormal findings. Plain abdominal radiographs were normal. There were no abnormal findings in urine cytology or cystoscopic examination. Computed tomography (CT) showed a 2.7×1.5 cm diffuse, noncalcified, moderately infiltrating mass with ill-defined lobulated walls located at the right common iliac vessel level (Fig. 1). The mass was located in the right ureter anteriorly, and mild focal enhancement, wall thickening, and luminal narrowing were found in the ureter (Fig. 1). The mass looked as if it compressed the right ureter, resulting in moderate hydroureteronephrosis above the affected level (Fig. 1). Retroperitoneal lymphadenopathy was not observed.
In a ureterorenoscopic examination, there was no intrinsic obstructive lesion such as a ureteral tumor causing ipsilateral hydronephrosis. A laparoscopic exploration was undertaken to debulk the tumor due to the patient's increasing symptoms. At the time of surgery, due to extensive adhesion of the ileocecal valve area and mass, the laparoscopic exploration was converted to open surgery to safely perform the tumor resection. We could not rule out the possibility of a malignant tumor before final histopathological confirmation of the affected lesion. The patient underwent extensive debulking of the mass including bowel segmentectomy with anastomosis and excision of the affected ureter. Then, end-to-end ureter anastomosis was performed, and a ureteral stent was deployed in the right urinary tract for decompression. A white wedge of tissue was obtained for pathological evaluation.
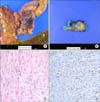
The specimen except of resected bowel segment was a 5.5×3×3 cm firm, poorly circumscribed mass. Upon sectioning, the cut surface of the mass revealed a white, whorled fibrous and trabecular appearance and ill-defined margins with the surrounding fat tissue (Fig. 2). Microscopically, the lesion was poorly circumscribed, with infiltration of the surrounding fat tissue, and was composed of cytologically bland, elongated, slender, spindle-shaped cells with collagenous stroma containing vessels of varying size. The cells were arranged in sweeping bundles and were admixed with a storiform growth pattern (Fig. 2). The cells lacked cytologic atypia or nuclear hyperchromasia and had vesicular nuclei with minute nucleoli. The mitotic figure was rare. In immunohistochemical staining, the spindle-shaped cells revealed nuclear β-catenin staining and focally positive staining for smooth muscle actin (SMA). However, the spindle-shaped cells were negative for C-kit (CD117), CD34, desmin, and S-100. Given the histopathological findings, a diagnosis of mesenteric fibromatosis was confirmed (Fig. 2).
The patient was discharged 5 days after surgery without any complications. The patient is attending follow-ups and shows no signs of abnormality or recurrence.
DISCUSSION
Fibromatosis can affect both superficial and deep parts of the body. Superficial fibromatosis can involve the face, neck, palms, feet, penis, shoulder, thigh, buttocks, or trunk. The deep variant can involve the abdominal wall, mesentery, retroperitoneum, mediastinum, or abdominal cavity. MF is an uncommon variant of deep fibromatosis and has only occasionally been reported [3]. Deep (or aggressive) fibromatosis is sometimes referred to as a desmoid tumor. A desmoid tumor is a very rare tumor that may or may not be part of a hereditary disease such as familial adenomatous polyposis. MF is a rare subtype involving the bowel mesentery (especially that of the small bowel) or retroperitoneum and comprises only 8% of all desmoid tumors [4]. Less common locations are the mesocolon and omentum [2]. The exact etiology of these lesions remains elusive. Various factors have been implicated by different researchers. Trauma, including previous surgery and estrogen exposure, may contribute to the formation of these tumors [5]. Genetic predisposition also plays a role, and patients with Gardner's syndrome have a much higher incidence of intra-abdominal fibromatosis. Although one-third of patients with Gardner's syndrome will have a desmoid tumor, only 2% of patients presenting with a desmoid tumor will be found to have Gardner's syndrome [6]. An infectious etiology is suggested by an association between human herpes virus and retroperitoneal fibromatosis in macaque monkeys [5]. The present case is peculiar in that MF presented in a woman without any of these factors.
Most patients are clinically asymptomatic, with little or no focal symptoms until later in the course of the disease, at which time they complain of pain, discomfort, constipation, vomiting, abdominal mass, weight loss, and symptoms due to organ compression [4]. Some complications that have been reported include small bowel obstruction and hydronephrosis [4]. In our case, the patient had intermittent right abdominal pain for a year, but there were no other symptoms, such as weight loss, emesis or a palpable mass.
Radiologically, metastatic carcinoma, abdominal lymphoma, intestinal carcinoids, peritoneal neoplasms, postoperative adhesions and retractile mesenteritis form the differential diagnoses [3]. A combination of CT for primary diagnosis and image-guided biopsies and magnetic resonance imaging (MRI) for periodic follow-up would be recommended in such patients, as both modalities have a large field of view and multiplanar and vascular imaging capabilities. Typical pathological features of MF include a well-circumscribed mass, often firmly attached to the small bowel; less often MF presents as an ill-defined and irregular mass. One study reported that the cut surface was a glistening pinkish-white in color and whorled. Histology revealed well-differentiated fibroblasts of uniform size with no evidence of mitotic activity, although there was infiltration of the surrounding tissue. Inflammatory infiltration was notably absent [3]. The pathologic confirmation of our case and the typical features of MF did not differ.
In confirming the pathologic diagnosis of MF, it can be difficult to distinguish MF from sclerosing mesenteritis that appears to be related to mesenteric panniculitis and mesenteric lipodystrophy. In such a case, immunohistochemical staining for beta-catenin can be useful, since MF consistently shows strong nuclear beta-catenin staining, whereas sclerosing mesenteritis does not express this antigen [7]. Distinguishing MF from gastrointestinal stromal tumors (GIST) of the mesentery is also of clinical significance due to their vastly different therapeutic and prognostic implications. Although the histopathologic features of MF and GIST can sometimes be confused, a differential diagnosis between MF and GIST can be carried out using immunohistochemical staining. In some studies, CD34 was common in GIST but was not detected in MF, and MF was consistently negative for KIT (CD117), whereas GIST was commonly positive. Nuclear beta-catenin staining characteristic of MF (but absent in GIST) is also a useful finding [7]. SMA, Desmin, and S-100 were not useful for distinguishing MF from GIST or sclerosing mesenteritis in previous studies [7].
Surgical excision remains the mainstay of MF therapy, with non-surgical modalities assuming a secondary role as high recurrence rates have been reported when surgery is used alone [5]. Indeed, it has been suggested that a recurrence could be caused by the surgical trauma itself. From the literature, it is evident that no single method of treatment is effective. The high recurrence rates after surgical excision may reflect the diffuse infiltrative growth pattern of desmoid tumors. As many mesenteric fibromatoses are unresectable because of their location and extent and because they are a cause of morbidity at the same time, alternative modes of therapy frequently become the primary treatment, to be followed by surgery if the tumor shrinks to within the limits of resection. Non-surgical modalities have also been used to reduce the recurrence rate. The use of hormonal therapy (i.e., tamoxifen), nonsteroidal anti-inflammatory drugs, and interferon as well as systemic chemotherapy may play an important role in the treatment of MF [8]. Post-operative radiation therapy is recommended and is often used for positive margins identified histologically, but its role is controversial [5]. In stable tumors and in those that diminish in size, no treatment may be necessary. Prognosis is good after complete excision. Local recurrences must also be treated by excision. A residual tumor left behind after surgery takes several years to spread before a second exploration is warranted. Subsequent surgery, however, is difficult due to extensive intraperitoneal adhesions created by previous surgical procedures. With each subsequent surgery, morbidity and mortality increase [3]. Extensive involvement of the abdominal viscera is the ultimate cause of death after several years [3].
Bilateral ureteral compression by multiple abdominal desmoid tumors in Gardner's syndrome has been described [9]. That case was managed by placement of a percutaneous nephrostomy tube and surgical removal of the desmoid tumors. Ng et al reported another case of hydronephrosis in mesenteric fibromatosis with Gardner's syndrome that was treated successfully with a cyclooxygenase-2 inhibitor [10]. In our case, the patient was treated by surgical excision of the mass, and she has been free of the tumor for 2 months.
MF and MF-induced ureteral stenosis is very rare. Although many mesenteric fibromatoses can be unresectable, patients with MF can undergo treatment successfully if we have an appropriate management plan including surgery and medical therapy.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download