Abstract
Pulmonary Langerhans cell histiocytosis is an uncommon diffuse cystic lung disease in adults. In rare cases, it can involve extrapulmonary organs and lead to endocrine abnormalities such as central diabetes insipidus. A 42-year-old man presented with polyphagia and polydipsia, as well as a dry cough and dyspnea on exertion. Magnetic resonance imaging of the hypothalamic-pituitary system failed to show the posterior pituitary, which is a typical finding in patients with central diabetes insipidus. This condition was confirmed by a water deprivation test, and the patient was also found to have type 2 diabetes mellitus. Computed tomographic scanning of the lungs revealed multiple, irregularly shaped cystic lesions and small nodules bilaterally, with sparing of the costophrenic angles. Lung biopsy through video-assisted thoracoscopic surgery revealed pulmonary Langerhans cell histiocytosis. On a follow-up visit, only 1 year after the patient had quit smoking, clinical and radiological improvement was significant. Here, we report an uncommon case of pulmonary Langerhans cell histiocytosis that simultaneously presented with diabetes insipidus and diabetes mellitus.
Pulmonary Langerhans cell histiocytosis (LCH) is a rare interstitial lung disease in adults that is a part of diseases caused by infiltration of Langerhans cell across multiple organs, such as the lungs, bones, skin, pituitary gland, and lymph nodes1. Among cases of multi-system LCH, simultaneous invasion of the lung and the pituitary gland is uncommon. Adult patients with pulmonary LCH may also develop extrapulmonary involvement in 17% of cases, such as diabetes insipidus in 5%-7% of patients23. Over 90% of LCH cases occurs in smokers, and the disease typically improves following smoking cessation4. Herein, we report a rare case of pulmonary LCH presenting with central diabetes insipidus and type 2 diabetes mellitus at diagnosis and improved after 1 year of smoking cessation.
A 42-year-old male was referred from a local clinic with a 1-year history of polydipsia and polyuria. He also complained of dry cough and dyspnea for 1 month. He was a current smoker with a 20 pack-year history and had been diagnosed with hypertension 4 years prior. The family history was unremarkable. On admission, the blood pressure was 147/81 mm Hg, heart rate was 120 per minute, respiratory rate was 16 per minute, and body temperature was 36.8℃. His height was 162 cm and the body weight was 82 kg (body mass index, 31.2). He appeared to be chronically ill. He did not present with pale conjunctiva, cyanosis, or clubbed fingers. Physical examination of the chest revealed fine crackle of both upper lung fields, and there was no audible cardiac murmur. The patient had no skin rash or palpable lymph nodes. The rest of the exam was unremarkable.
Complete blood count revealed a total leukocyte count 11,480/mm3 (neutrophil, 61.1%; lymphocyte, 28.1%; monocyte, 7.0%; eosinophil, 1.2%; basophil, 0.3%), hemoglobin 14.2 g/dL, hematocrit 42.1%, and platelet count 421,000/mm3. Serum chemistry demonstrated a total protein level of 7.1 g/dL, albumin 4.6 g/dL, blood urea nitrogen 5.5 mg/dL, creatinine 0.83 mg/dL, aspartate transaminase 37 U/L, alanine transaminase 47 U/L, total bilirubin 0.34 mg/dL, and C-reactive protein 0.62 mg/dL.
The patient was also underwent an endocrinologic evaluation of the anterior and posterior pituitary including the following: thyroid stimulating hormone, 1.146 µIU/mL (0.35-5.50 µIU/mL); total T3, 1.40 ng/mL (0.60-1.81 ng/mL); free T4, 1.45 ng/dL (0.83-1.76 ng/dL); growth hormone, 0.102 ng/mL (<13.0 ng/mL); insulin-like growth factor-1, 182.0 ng/mL (101-267 ng/mL); prolactin, 4.98 ng/mL (2.1-17.7 ng/mL); lutenizing hormone, 5.23 mIU/mL (1.5-9.3 mIU/mL); follicular stimulating hormone, 9.69 mIU/mL (1.4-18.1 mIU/mL); testosterone, 3.53 ng/mL (2.8-11.0 ng/mL); cortisol, 25.14 µg/dL (4.3-22.4 µg/dL); adrenocorticotrophic hormone, 54.6 pg/mL (12.0-60.0 pg/mL); and antidiuretic hormone, 4.23 pg/mL (<6.7 pg/mL). Osmolality of the urine was 87 mmol/kg and serum osmolality was 280 mmol/kg. Urine specific gravity was 1.001 (1.015-1.030). Water deprivation test and a vasopressin response trial revealed a diagnosis of diabetes insipidus (Table 1). For evaluating whether the cause of central diabetes insipidus was autoimmunity, IgG4 subclass antibody was measured and its level revealed 208.0 mg/L (30-2,010 mg/L). Fasting plasma glucose was 145 mg/dL and hemoglobin A1c was 7.0%.
Magnetic resonance imaging of the hypothalamic-pituitary system revealed neither infundibular enlargement nor space occupying lesions of the pituitary or hypothalamic glands. But, the hyperintense signal of the posterior pituitary on T1-weighted images was not detectable which is typically absent in LCH patients with central diabetes mellitus (Figure 1).
Chest radiograph was notable for reticular opacities in bilateral upper and middle lung fields. On computed tomography of the chest, numerous irregularly shaped cysts and centrilobular nodules were observed in the bilateral lung fields. These findings were more prominent in the upper lobes but were not visible on both costophrenic angles (Figure 2A).
The patient underwent bronchoalveolar lavage, and the lavage specimen was analyzed for further evaluated. The leukocyte count of the lavage was 342/µL, and the differential count was reported as lymphocyte 17%, polymorphic neutrophil 4%, basophil 2%, and monocyte 1%. Acid-fast bacilli staining and culture of the sputum, fungus culture, and polymerase chain reaction for Mycobacterium tuberculosis were all negative.
On pulmonary function testing, the forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio was 82%, with an FEV1 of 3.04 L (93% of predicted value) and FVC of 3.81 L (93% of predicted value). Plethysmography was performed, showing residual volume of 0.68 L (43% of predicted value), and a total lung capacity of 4.49 L (84% of predicted value). Diffusing capacity for carbon monoxide was 15.4 mL/mm Hg/min, which was 60% of the predicted value.
Video-assisted thoracoscopic surgery was performed to obtain a biopsy specimen of the lung in the right upper lobe. On histologic examination, multiple cystic spaces were visible upon a grayish-white granular layer. Immunohistochemical evaluation revealed strong positivity for CD1 and S-100 protein, suggesting diffuse infiltration of the Langerhans cell, confirming the diagnosis of LCH (Figure 3).
Involvement of additional systems was evaluated using radiographic examination. A skeletal survey including skull, vertebral, and pelvic series revealed no abnormality. Abdominal computed tomography scan revealed no hepatic or splenic abnormalities. A positron emission tomography-computed tomography was performed to identify lesions potentially missed by other modalities but did not reveal further systemic involvement.
The patient was diagnosed with pulmonary LCH complicated by endocrinologic dysfunction with central diabetes insipidus and diabetes mellitus. Systemic therapy was considered in this case. The patient was started on oral methylprednisolone 1 mg/kg. However, the patient developed profound muscle weakness after 2 weeks of steroid therapy, and, for these reasons, it was withdrawn. The patient stopped smoking and his symptoms gradually improved following smoking cessation. After 1-year follow-up, computed tomography scan of the chest revealed interval decrease in size and thickness of the cysts found at presentation (Figure 2B). Interval pulmonary function testing revealed significant improvement, with an FEV1/FVC of 76%, FEV1 of 3.14 L (97% of predicted value), FVC of 4.11 L (100% of predicted value), residual volume of 1.25 L (78% of predicted value), total lung capacity of 5.36 L (100% of predicted value), and diffusing capacity for carbon monoxide of 15.3 mL/mm Hg/min, 60% of predicted value. In contrast to these findings, magnetic resonance imaging of the brain revealed no interval change. The patient was continued on desmopressin 0.6 mg for diabetes insipidus and metformin 500 mg for diabetes mellitus.
The patient in our case was diagnosed with pulmonary LCH that simultaneously presented with central diabetes insipidus and type 2 diabetes mellitus in the middle age. After 1-year of smoking cessation, the patient had demonstrated significant clinical and radiologic improvement. In cases of multisystem LCH, systemic therapy should be considered for treatment with vinblastine/prednisolone, cytarabine, or 2-CDA5. Twenty-six percent of patients with multisystem involvement progress despite treatment4. Prognosis varies widely, from spontaneous remission to high risk of mortality due to disease progression. Poor prognostic factors include systemic symptoms, old age, female sex, invasion of the central nervous system, or costophrenic angles, decreased diffusion capacity of the lungs, and repeated pneumothorax1. While the available treatment options differ among isolated pulmonary LCH, single system LCH, and multisystem LCH, smoking cessation is the most important treatment in all forms of LCH. Despite the lack of reliable data on the association between smoking cessation and the prognosis of LCH due to its rarity and high rate of spontaneous remission, numerous case reports have described that smoking cessation can bring significant improvements in the disease6. There are no clinical study-based data supporting corticosteroid therapy for pulmonary LCH. However, systemic steroid therapy (1 mg/kg/day for 1 month, followed by tapering doses over months) may be administered to patients who do not respond after smoking cessation for treatment5. The treatment of pulmonary LCH may aided by the use of systemic steroids and/or systemic chemotherapy, while the use of methotrexate, azathioprine, thalidomide, 2-CDA, and systemic chemotherapy may be considered in conjunction with smoking cessation in multisystem disease1. Although improvements in extrapulmonary LCH may not be associated with smoking cessation, smoking is an important prognostic factor for pulmonary LCH, and smoking cessation resulted in disease improvement in our case. In addition to smoking cessation, we considered steroid therapy for treatment in our patient. However, we could not maintain steroid therapy because he developed steroid-induced myopathy after short-term high dose corticosteroid administration. Two-week period of steroid therapy was too short to improve the lung lesions of disease. In case of progressive pulmonary LCH despite steroid therapy, systemic chemotherapy may be considered with 2-CDA5. The current patient had subsequently shown the clinical and radiologic improvement with only smoking cessation every 3 months of follow-up. Therefore, we did not consider using other chemotherapeutic agents for further treatment in our patient.
LCH associated pituitary dysfunction, characterized by diabetes insipidus, is found in 14% of patients with LCH4 and 7% of adult patients with pulmonary LCH have diabetes insipidus23. A recent study demonstrated that adults patients with LCH have a high prevalence of abnormalities of glucose metabolism presenting either as impaired glucose tolerance or type 2 diabetes mellitus7. There are a few case reports of LCH associated with diabetes insipidus and diabetes mellitus89. Our patient also had pathologically-proven pulmonary LCH combined with diabetes insipidus and type 2 diabetes mellitus at diagnosis, simultaneously.
Clinical features of LCH are mostly mild, with patients complaining of dyspnea, cough, bone pain, abnormal growth of soft tissue, skin rash, pruritus, thirst, and lymphadenitis. Other less frequent symptoms of LCH include fatigue, general weakness, nocturnal chilling, nausea, and fever4. The disease is capable of invading distant organs, including the bone marrow, liver, spleen, or central nervous system, which can be refractory to therapy and become fatal5.
The disease can also involve the hypothalamus-pituitary axis, which then mostly manifests as diabetes insipidus. Therefore, LCH patients complaining of polyphagia and polydipsia must be evaluated for diabetes insipidus by evaluating the urine and plasma osmolality and by performing the water deprivation test and magnetic resonance imaging of the head5. In Koreans, several cases of LCH involving the hypothalamicpituitary axis and the lungs have been reported1011. However, only 1 case presented with pulmonary LCH and central diabetes insipidus as the initial manifestation and was of adult onset. In that case, the patient was not treated with chemotherapeutic agents and his disease did not progress during the 4-year follow-up.
Magnetic resonance imaging of the hypothalamic-pituitary system in patients with LCH may show infundibular enlargement, pituitary infiltration, empty sella, and hypothalamic involvement12. In addition, the hyperintense signal of the normal posterior pituitary gland, the so-called "bright spot" is typically lacking13. This finding can be detected in 82%-100% of patients with LCH presenting with central diabetes insipidus1213. Therefore, the patients with LCH in whom central diabetes insipidus is clinically suspicious should be evaluated for the involvement of pituitary glands using magnetic resonance imaging.
Diabetes insipidus in patients with LCH generally does not respond to any LCH-directed treatment and requires long-term replacement therapy with desmopressin14.
Computed tomography scan of the chest may show nodular, cystic, or mixed patterns involving the lung parenchyma, which is in keeping with the radiologic findings of our case, which demonstrated numerous, bilateral irregularly-shaped cysts and centrilobular nodules, predominantly involving the upper lobes15. Because of the very low incidence of LCH in adults, few reports demonstrate the usefulness of positron emission tomography-computed tomography for identification of lesions missed by other modalities and assessment of responses to therapy1617.
In conclusion, we report the clinical experience and management of a patient who simultaneously presented with pulmonary LCH, central diabetes insipidus, and type 2 diabetes mellitus and developed significant clinical and radiologic improvement following one year of smoking cessation.
Figures and Tables
Figure 1
Magnetic resonance imaging of the hypothalamic-pituitary system. The hyperintense signal of the posterior pituitary on the T1-weighted image could not be detected. Lack of this bright spot (arrow) is typical of central diabetes insipidus.
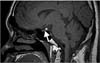
Figure 2
Computed tomographic scans of the chest. (A) Multiple irregular cysts and centrilobular nodules can be seen in both lung fields, with sparing of the costophrenic angles. (B) Follow-up scan at 1-year after the patient stopped smoking. The size and thickness of the irregular cysts are reduced, and both lungs show a decrease in the number of centrilobular nodules.

Figure 3
Langerhans cell histiocytosis. (A) The cut surface of wedge-resected lung shows numerous cystic spaces with whitish gray stellate fibrous scars. (B) Multiple cystic spaces are evident, with diffuse thickening, cellular infiltration, and fibrous tissue (H&E stain, ×12.5). (C) The cytoplasm of the infiltrated cells is pale and eosinophilic, and the nuclei are grooved or infolded (H&E stain, ×400). (D) Immunohistochemical staining of the proliferating cells is diffuse and strongly positive for CD1a (×400).
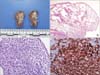
Table 1
Results of water deprivation test and treatment with vasopressin

References
1. Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans'-cell histiocytosis. N Engl J Med. 2000; 342:1969–1978.
2. Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary Langerhans'-cell histiocytosis in adults. N Engl J Med. 2002; 346:484–490.
3. Kim C, Jeong SH, Shim JJ, Cha SI, Son C, Chung MP, et al. Clinical features of pulmonary Langerhans cell histiocytosis in Korea. Tuberc Respir Dis. 2009; 66:98–103.
4. Howarth DM, Gilchrist GS, Mullan BP, Wiseman GA, Edmonson JH, Schomberg PJ. Langerhans cell histiocytosis: diagnosis, natural history, management, and outcome. Cancer. 1999; 85:2278–2290.
5. Girschikofsky M, Arico M, Castillo D, Chu A, Doberauer C, Fichter J, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis. 2013; 8:72.
6. Mogulkoc N, Veral A, Bishop PW, Bayindir U, Pickering CA, Egan JJ. Pulmonary Langerhans' cell histiocytosis: radiologic resolution following smoking cessation. Chest. 1999; 115:1452–1455.
7. Alexandraki KI, Makras P, Protogerou AD, Dimitriou K, Stathopoulou A, Papadogias DS, et al. Cardiovascular risk factors in adult patients with multisystem Langerhans-cell histiocytosis: evidence of glucose metabolism abnormalities. QJM. 2008; 101:31–40.
8. Thai AC, Sng I, Suri R, Cheah JS. Disseminated histiocytosis X with diabetes insipidus and diabetes mellitus in an adult female (histiocytosis with DI and DM). Ann Acad Med Singapore. 1988; 17:294–298.
9. Rowntree LG, Poppiti RJ. Diabetes inspidius, diabetes mellitus, and insulin resistance with histiocytosis. J Am Med Assoc. 1954; 156:310–312.
10. Hong ES, Ohn JH, Kim JH, Hwang-Bo YK, Kim JJ, Kwon JH, et al. Clinical characteristics of Langerhans cell histiocytosis with hypothalamo-pituitary involvement. Endocrinol Metab. 2011; 26:38–43.
11. Choi JE, Lee HR, Ohn JH, Moon MK, Park J, Lee SJ, et al. Adult multisystem langerhans cell histiocytosis presenting with central diabetes insipidus successfully treated with chemotherapy. Endocrinol Metab. 2014; 29:394–399.
12. Makras P, Samara C, Antoniou M, Zetos A, Papadogias D, Nikolakopoulou Z, et al. Evolving radiological features of hypothalamo-pituitary lesions in adult patients with Langerhans cell histiocytosis (LCH). Neuroradiology. 2006; 48:37–44.
13. Grois N, Prayer D, Prosch H, Minkov M, Potschger U, Gadner H. Course and clinical impact of magnetic resonance imaging findings in diabetes insipidus associated with Langerhans cell histiocytosis. Pediatr Blood Cancer. 2004; 43:59–65.
14. Arico M, Girschikofsky M, Genereau T, Klersy C, McClain K, Grois N, et al. Langerhans cell histiocytosis in adults: report the International Registry of the Histiocyte Society. Eur J Cancer. 2003; 39:2341–2348.
15. Moore AD, Godwin JD, Muller NL, Naidich DP, Hammar SP, Buschman DL, et al. Pulmonary histiocytosis X: comparison of radiographic and CT findings. Radiology. 1989; 172:249–254.
16. Hansen NJ, Hankins JH. Pulmonary langerhans cell histiocytosis: PET/CT for initial workup and treatment response evaluation. Clin Nucl Med. 2015; 40:153–155.
17. Lee HJ, Ahn BC, Lee SW, Lee J. The usefulness of F-18 fluorodeoxyglucose positron emission tomography/computed tomography in patients with Langerhans cell histiocytosis. Ann Nucl Med. 2012; 26:730–737.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download