Abstract
Malignant mesothelioma is a rare malignant neoplasm that arises from mesothelial surfaces of the pleural cavity, peritoneal cavity, tunica vaginalis, or pericardium. Typically, pleural fluid cytology or closed pleural biopsy, surgical intervention (video thoracoscopic biopsy or open thoracotomy) is conducted to obtain pleural tissue specimens. However, endobronchial lesions are rarely seen and cases diagnosed from bronchoscopic biopsy are also rarely reported. We reported the case of a 77-year-old male who was diagnosed as malignant mesothelioma on bronchoscopic biopsy from obstructing masses of the endobronchial lesion.
Malignant mesothelioma is a rare cancer associated with asbestos exposure that is estimated to occur in approximately 2,500 people in the United States every year12. In Korea, the incidence of malignant mesothelioma is approximately 1-2 persons per million3. Malignant mesothelioma most commonly occurs in pleura, but also in lining of other sites (e.g., peritoneum, pericardium, and tunica vaginalis testis)456. Malignant mesothelioma is a mesodermally derived neoplastic disease and it is rare that originates in other than pleura, especially lung parenchyme or endobronchial mass via direct invasion7.
Patients with suspected malignant pleural mesothelioma have symptoms such as dyspnea and chest pain and can also have pleural effusion, cough, chest wall mass, weight loss, fever, and sweating8. The recommended initial evaluation for suspected malignant pleural mesothelioma includes chest computed tomography (CT) or magnetic resonance imaging, 18fluoro-2-deoxyglucose positron emission tomography (PET). For tissue diagnosis, thoracentesis for cytology and closed pleural biopsy, thoracoscopic biopsy and open lung biopsy could be done91011. Rarely, there are few reports of malignant pleural mesothelioma diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration1213.
We reports a case of malignant pleural mesothelioma that was diagnosed by bronchoscopic biopsy of endobronchial mass mimicking lung cancer.
A 77-year-old male visited at a secondary hospital with history of dyspnea. He had a previous history of rectal cancer 20 years ago, complete remission state after Mile's operation and hypertension which was well controlled with losartan. He had been smoked about 55 pack-years. Large pleural effusion of right hemithorax was found on chest radiograph. Thoracentesis and closed pleural biopsy were performed. Pleural fluid analysis yielded exudates (pleural fluid lactate dehydrogenase and total protein were 920 IU/L and 4.6 g/dL, respectively) and pleural fluid culture and culture for acid-fast bacilli were negative. The results of cytology and pleural biopsy were suggestive of malignancy, but primary site was undetermined. PET-CT and chest CT were done, chest CT showed peribronchial soft tissue thickening in right middle lobe (RML), right lower lobe (RLL), that result in severe bronchial narrowing and collapse of RML and RLL, and large amount of pleural effusion. But there is no definite pleural thickening on chest CT. PET-CT showed high glucose uptake in RML, RLL, and pleura (Figures 1, 2).
He transferred to our hospital to evaluate primary site of suspected metastatic pleural effusion. On 12 March 2014, bronchoscopy was performed and we found infiltrating masses of medial segmental bronchus of RML and entry of anterior segment of RLL, and then bronchoscopic biopsy was done.
Bronchoscopic biopsy and immunohistochemical stain revealed malignant mesothelioma (Figure 3). And we reviewed slides of pleural biopsy performed at the other hospital, it was also revealed as malignant mesothelioma. On immunohistochemical stain of bronchoscopic biopsy specimen, Wilms tumor 1 (WT-1), calretinin, cytokeratin 5/6 (CK5/6), p63 were all positive and napsin-A, thyroid transcription factor 1 (TTF-1) were negative. Those of pleural biopsy specimen, calretinin, D2-40, CK5/6, and p63 were positive, and napsin-A and TTF-1 were negative (Figure 3).
We reviewed in depth his previous jobs and residences and found he worked in construction areas from 1991 to 2003, it was presumed that he had a lot of exposure to asbestos in those days.
He was treated with combination chemotherapy of cisplatin and pemetrexed every 3 weeks. After 2 cycle of pemetrexed and cisplatin combination chemotherapy, pleural effusion was decreased on chest radiograph and dyspnea was improved. However, cough was persist, so we performed bronchoscopy again, and found that obstructive infiltrating mass at entry of anterior segment of RLL, then biopsy was done. The result also revealed it was malignant mesothelioma with squamous differentiation, calretinin, p63 were positive and WT-1 and D2-40 were negative (Figure 3).
Also after four cycle of chemotherapy, chest CT showed partial response, nodules of pleura were decreased in size and peribronchial soft tissue thickening was also decreased 4.0×2.3 cm to 3.3×1.3 cm (Figure 4).
We reported unusual case of malignant mesothelioma that had endobronchial masses and was diagnosed by bronchoscopic biopsy, however, pleural thickening was not predominant exceptionally.
Malignant pleural mesothelioma is a neoplasm that can arise from the mesothelial surface of the pleura and tends to be locally invasive and has poor prognosis, with median overall survival range between 9 to 17 months2. Malignant pleural mesothelioma occurs predominantly in men and risk increases with age (45 to 85 years). A large proportion of patients diagnosed at earlier ages have a history of exposure of asbestos.
The differential diagnosis of malignant pleural mesothelioma includes both benign and malignant process. Chronic organized empyema is often similar finding, such as dense pleural thickening and large pleural effusion. And it is also difficult to distinguish from metastatic adenocarcinoma of pleura. For accurate diagnosis of mesothelioma, histopathological examination should be performed.
There are three main histologic subtypes of mesothelioma: epithelioid, biphasic, and sarcomatoid. For definitive diagnosis of malignant mesothelioma, it is required that a workup including immunohistochemistry and in some cases, histochemical stains for mucin. The primary differential diagnosis for epithelioid mesothelioma in the pleura is with metastatic lung carcinoma and is heavily reliant on immunohistochemistry using a battery of mesothelial and carcinoma markers. For instance, calretinin, WT-1 are usually positive in malignant pleural mesothelioma and p63, MOC-31, BG8, and Ber-EP4 are usually positive in lung squamous carcinoma14.
When we followed up bronchoscopy, in hematoxylin and eosin histology, keratin pearls were showed. And we had doubts about in case of squamous cell carcinoma was misdiagnosed as malignant mesothelioma. However, immunohistochemistry results demonstrated malignant mesothelioma, and neoplasm's size had decreased after pemetrexed and cisplatin combination chemotherapy. As a result, it has low possibility of misdiagnosis.
Bronchoscopic biopsy is not the diagnostic choice of malignant mesothelioma, even though some literatures of biopsy using bronchoscopy is obtaining mediastinal lymph node by using endobronchial ultrasound1213.
DellaGiustina et al.15 described 3 patients with malignant pleural mesothelioma with who had positive bronchial cytology. And the authors concluded, in rare cases, pleural mesothelioma cells are shed within the airway lumina and can be detected in bronchoscopic cytology specimens. We could assume it could be explainable hypothesis of our case.
In this case, the endobronchial mass was observed by bronchoscopy and biopsy of this mass showed malignant mesothelioma. Moreover, there was no report about direct bronchoscopic biopsy of malignant mesothesioma.
Accordingly, bronchoscopy would be considered as a way to acquire tissue specimen in malignant pleural mesothelioma, although it is less invasive than thoracoscopy or thoracotomy.
Figures and Tables
Figure 1
Large amount of pleural effusion with passive collapse of right middle lobe and right lower lobe on chest radiography (A) and peribronchial soft tissue thickening in right middle lobe and right lower lobe on chest computed tomography (B, C).
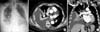
Figure 2
Positron emission tomography computed tomography showed multiple high uptake lesions (A-C), and bronchoscopic biopsy was obtained from anterobasal segment of right lower lobe (D-F).
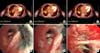
Figure 3
(A-D) Initial bronchoscopic biopsy findings of papillary clusters of epithelioid cells, and positive staining with anti-calretinin antibody, cytokeratin 5/6, and Wilms tumor 1 stain in most of the neoplastic cells. (E-H) Pleural biopsy findings of neoplastic cells positive for anti-calretinin antibody, and cytokeratin 5/6, and definitive Wilms tumor 1. (I-L) Follow-up bronchoscopic biopsy findings of neoplastic cells positive for anti-calretinin antibody, and negative for p63, Wilms tumor 1 stain.

References
1. Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol. 2009; 39:576–588.
2. Tsao AS, Wistuba I, Roth JA, Kindler HL. Malignant pleural mesothelioma. J Clin Oncol. 2009; 27:2081–2090.
3. Kim HR, Ahn YS, Jung SH. Epidemiologic characteristics of malignant mesothelioma in Korea. J Korean Med Assoc. 2009; 52:449–455.
4. Carteni G, Manegold C, Garcia GM, Siena S, Zielinski CC, Amadori D, et al. Malignant peritoneal mesothelioma. Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer. 2009; 64:211–218.
5. Mirarabshahii P, Pillai K, Chua TC, Pourgholami MH, Morris DL. Diffuse malignant peritoneal mesothelioma: an update on treatment. Cancer Treat Rev. 2012; 38:605–612.
6. Chekol SS, Sun CC. Malignant mesothelioma of the tunica vaginalis testis: diagnostic studies and differential diagnosis. Arch Pathol Lab Med. 2012; 136:113–117.
7. Sterman DH, Albelda SM. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology. 2005; 10:266–283.
8. Gadgeel SM, Pass HI. Novel combinations using pemetrexed in malignant mesothelioma. Clin Lung Cancer. 2004; 5:Suppl 2. S61–S66.
9. Scherpereel A, Astoul P, Baas P, Berghmans T, Clayson H, de Vuyst P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010; 35:479–495.
10. Kao SC, Yan TD, Lee K, Burn J, Henderson DW, Klebe S, et al. Accuracy of diagnostic biopsy for the histological subtype of malignant pleural mesothelioma. J Thorac Oncol. 2011; 6:602–605.
11. Greillier L, Cavailles A, Fraticelli A, Scherpereel A, Barlesi F, Tassi G, et al. Accuracy of pleural biopsy using thoracoscopy for the diagnosis of histologic subtype in patients with malignant pleural mesothelioma. Cancer. 2007; 110:2248–2252.
12. Hamamoto J, Notsute D, Tokunaga K, Sasaki J, Kojima K, Saeki S, et al. Diagnostic usefulness of endobronchial ultrasoundguided transbronchial needle aspiration in a case with malignant pleural mesothelioma. Intern Med. 2010; 49:423–426.
13. Kang B, Kim MA, Lee BY, Yoon H, Oh DK, Hwang HS, et al. Malignant pleural mesothelioma diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Tuberc Respir Dis. 2013; 74:74–78.
14. Husain AN, Colby T, Ordonez N, Krausz T, Attanoos R, Beasley MB, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2013; 137:647–667.
15. DellaGiustina D, Falconieri G, Bonifacio-Gori D, Zanconati F, DiBonito L, Pizzolitto S. Bronchial cytology in pleural mesothelioma: a report of 3 positive cases, including 1 diagnosed initially on bronchial brushings. Acta Cytol. 2003; 47:1017–1022.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download