Abstract
Malignant rhabdoid tumor was first discovered in the kidney, and rhabdoid tumor of the lung was first reported in 1995. These were included as the variants of large-cell carcinoma, according to the 1999 World Health Organization classification of lung tumors. The rhabdoid tumor of the lung exhibits aggressive biological behavior and has a poor prognosis, and only a few reports of this tumor exist. We report a case of lung carcinoma with a rhabdoid phenotype, initially misdiagnosed as an aspergilloma, in a 48-year-old man who presented with recurrent hemoptysis. The chest computed tomography scans showed a huge consolidative lesion with an air crescent sign in the left upper lung and no contrast-enhancing lesion. An aspergilloma was diagnosed by the radiologist. However, after surgical excision and pathological examination, rhabdoid carcinoma was diagnosed. A surgical resection helps to make it possible to pathologically distinguish a malignancy from an aspergilloma.
Rhabdoid tumor was first described as a renal neoplasm consisting of cells resembling neoplastic rhabdomyoblasts in 19781 in a pediatric population. Since then, several renal and extrarenal tumors containing rhabdoid cells have been reported. The first case of a rhabdoid tumor in the lung was reported as a neuroendocrine carcinoma with rhabdoid phenotype2 in 1995. Rhabdoid tumors of the lung have been included as a variant of large cell carcinoma according to the World Health Organization classification of lung tumors since 19993. It is important to recognize tumors with rhabdoid cells because they have aggressive biologic behavior and poor prognosis4. There have been a few cases of lung carcinoma with a rhabdoid phenotype in the international literature.
We experienced a case of lung carcinoma with a rhabdoid phenotype mimicking an aspergilloma, with recurrent hemoptysis as a symptom, and we report this case with a review of the literatures.
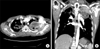
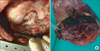
A 48-year-old male visited our hospital, complaining of recurrent abundant hemoptysis for 2 months. He was a smoker with a 40 pack-year smoking history and had been taking medications for diabetes for 5 years. He did not have a family history of malignancy or immunosuppressive disease. He had a history of pulmonary tuberculosis 3 years ago and had taken an anti-tuberculosis medication for 6 months. On admission, he presented with a blood pressure of 110/70 mm Hg, a heart rate of 80 beats per minute, a respiratory rate of 20 breaths per minute, and a body temperature of 36.8℃. Physical examination of the chest revealed crackles in both lungs. Lymph nodes in the neck could not be palpated. The complete blood count showed a white blood cell count of 14,200/mm3 (neutrophils, 78.4%; lymphocytes, 16.2%; monocytes, 5.1%; and eosinophils, 0.2%), hemoglobin level of 7.0 g/dL and platelet count of 665,000/mm3. Routine blood chemistry and tumor markers were within normal limits except for C-reactive protein, which was 15.5 mg/dL. Chest X-ray and computed tomography (CT) scans of the chest revealed a consolidative lesion, which had high internal attenuation with bubble lucency, and a solid mass of the left upper lung (Figure 1B). Also there was a round mass with soft-tissue opacity within a lung cavity of the right upper lung. In the left upper lung, the mass was separated from the wall of the cavity by an airspace of variable shape and size indicative of an "air crescent" sign, and there was no contrast-enhancement (Figure 1A). There was no evidence of mediastinal or hilar lymphadenopathy elsewhere radiologically. The clinical diagnosis was a fungal ball with hematoma. The patient underwent bronchial artery embolization twice for persistent hemoptysis. After this, fiberoptic bronchoscopy for pre-operation evaluation of the exact site of bleeding was planned. Fiberoptic bronchoscopy showed that some blood clots with active bleeding were present in the left upper lobe apicoposterior segment (especially in the posterior subsegment). Because of the uncontrolled hemoptysis, the patient underwent left upper lobectomy and decortication. Video-assisted thoracoscopic surgery showed that there was a 20×12×8-cm-sized mass (Figure 2A) and a 8×7-cm-sized cavitary lesion in the left upper lobe (Figure 2B).
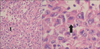
Pathologic examination of the biopsy revealed extensive central necrosis and a solid area without any evidence of fungal involvement. On immunohistochemical staining, the tumor cells showed a negative reaction for cytokeratin, vimentin, actin, desmin, S-100, CD34, CD68, D2-40, calretinin, and p63, except for a focally positive reaction to epithelial membrane antigen. The tumor cells had a large nucleus with atypism and focally eccentric eosinophilic cytoplasm (Figure 3). The tumor was diagnosed as an undifferentiated malignant tumor of the rhabdoid phenotype because of the poor differentiation. A brain magnetic resonance imaging and positron emission tomography-computed tomography scan demonstrated no metastatic lesion and we made a plan to follow up the patient with CT scanning in the outpatient department.
Rhabdoid tumor was reported as a renal neoplasm consisting of cells resembling neoplastic rhabdomyoblasts for the first time in 1978, in a pediatric population1. The term 'rhabdoid' in rhabdoid tumor refers to rhabdomyosarcoma because of the morphological similarity of the cells in these two types of tumors4. While most renal rhabdoid tumors are found in childhood, extrarenal rhabdoid tumors are observed in a broad range of age groups5. Additionally, almost all of cases of rhabdoid tumoroccur in the kidney, whereas extrarenal rhabdoid tumors appear in a variety of other locations such as soft tissue, liver, uterus, brain, prostate, vulva, urinary bladder, intestine and stomach, and recently the lung5. Rhabdoid tumor in the lung is considered as a variant of large cell carcinoma according to the World Health Organization classification of lung and pleural tumors of 19993. Rhabdoid tumors of the lung are rare6 and a pure rhabdoid phenotype is even more rare3. Cavazza et al.6 and Shimazaki et al.7 proposed a diagnostic criterion for lung tumors with a rhabdoid phenotype; at least 10% of the tumor cell population shouldconsist of rhabdoid cells. But they did not explain why a rate of 10% rhabdoid cells was chosen as the cut-off point8. Also, they suggested that tumors consisting of more than 10% rhabdoid cells were more aggressiveg6,7 whereas the presence of less than 5% rhabdoid cells had a negligible effect on prognosis7. However, the World Health Organization classified pulmonary neoplasms containing >10% of rhabdoid cells as a variant of large cell carcinoma with a rhabdoid phenotype (LCC-RP) in 20049. Histologically, the rhabdoid cell is described as being a large cell with a large eccentric nucleus, a central macronucleolus and a rounded eosinophilic cytoplasmic inclusion within abundant cytoplasm10. Clinically, rhabdoid lung carcinoma commonly occurs in middle-aged to elderly male smokers. And since it shows aggressive biological behavior, most cases of rhabdoid carcinoma are found in the advanced stage7. The tumor has an unfavourable prognosis because of its rapid and fatal progression. In our patient's case, the tumor brought about persistent hemoptysis, which probably led to the early diagnosis and subsequent complete resection of the tumor. In our case, the chest CT showed a huge consolidative mass with high internal attenuation. The mass was separated from the wall of the cavity by an airspace, like an "air crescent" sign, and no contrast-enhanced lesion was seen. Moreover, the mass had a large area of central necrosis, and there was no lymph node involvement. The mass involved the visceral pleura but not the parietal pleura. The radiologist reported the mass as an aspergilloma. But after the operation, the mass was diagnosed as a stage IIB (T3N0M0) malignancy. Park et al.11 have suggested that there are five points to consider when making a differential diagnosis between an aspergilloma and a cavitating lung cancer on contrast-enhanced CT. These are 1) a determination of whether the cavitary lesion is contrast-enhanced or not, 2) an assessment of the dependent location of a mural nodule within the cavity, 3) the presence of adjacent bronchiectasis, and 4) the loss of volume of the involved pulmonary lobe. Additionally, 5) wall thickness of the cavity could be helpful for making a differential diagnosis between an intracavitary aspergilloma and a cavitating lung cancer11. But in our case, it was difficult to diagnose the pulmonary cavitating lesion as a carcinoma using the radiologic findings. Therefore, surgical resection was performed, which made it possible to make a pathological diagnosis and distinguish the malignancy from an aspergilloma, and give appropriate treatment to the patient.
Also, we must consider the possibility of a malignancy arising from a cavitary pulmonary tuberculosis scar because the risk of lung cancer arising subsequent to a pulmonary tuberculosis infection is significantlyhigh12. Therefore, it is important to check radiological images of the lesion after anti-tuberculosis treatment is given to the patient, but in our case, we could not do that since the patient refused for personal reasons. After the operation, we planned to give the patient chemoradiotherapy but there is still no established effective standard regimen. Also there is no general consensus about the typical findings of large cell carcinoma with the rhabdoid phenotype. We believe that more cases of rhabdoid lung carcinoma need to be accumulated before this determination can be made. And we suggest that even if the LCC-RP is resected completely in the early stage, the patient must be followed up more carefully than other patients with classic large cell carcinoma13.
In conclusion, we herein report a case of rhabdoid lung carcinoma. Because patients with rhabdoid lung carcinoma may show rapid deterioration, an early diagnosis of this lesion needs to be made. We think that surgical resection makes it possible to make a pathological diagnosis and give appropriate treatment, by distinguishing between a malignancy and an aspergilloma.
Figures and Tables
Figure 1
Computed tomography scan of the chest shows a huge (8.3-cm extent) consolidative lesion with air crescent sign (A), internal high attenuation, and bubble lucency in the left apical lung (B).
References
1. Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors: results from the First National Wilms' Tumor Study. Cancer. 1978; 41:1937–1948.
2. Colby TV, Koss MN, Travis WD. Tumors of the lower respiratory tract. Atlas of tumor pathology. Fascicle 13. Washington, DC: AFIP;1995. p. 311.
3. Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. Histological typing of the lung and pleural tumours. Berlin: Springer-Verlag;1999. p. 40–42.
4. Tamboli P, Toprani TH, Amin MB, Ro JS, Ordonez NG, Ayala AG, et al. Carcinoma of lung with rhabdoid features. Hum Pathol. 2004; 35:8–13.
5. Parham DM, Weeks DA, Beckwith JB. The clinicopathologic spectrum of putative extrarenal rhabdoid tumors. An analysis of 42 cases studied with immunohistochemistry or electron microscopy. Am J Surg Pathol. 1994; 18:1010–1029.
6. Cavazza A, Colby TV, Tsokos M, Rush W, Travis WD. Lung tumors with a rhabdoid phenotype. Am J Clin Pathol. 1996; 105:182–188.
7. Shimazaki H, Aida S, Sato M, Deguchi H, Ozeki Y, Tamai S. Lung carcinoma with rhabdoid cells: a clinicopathological study and survival analysis of 14 cases. Histopathology. 2001; 38:425–434.
8. Saini G, Kumar M, Julka PK, Puri T, Sharma M, Rath GK. Rhabdoid variant of lung cancer: clinicopathological details of a case and a review of literature. J Cancer Res Ther. 2009; 5:54–57.
9. Travis WD, Brambilla E, Muller-Hermlink HK, Harris CC. World Health Organization classification of tumors: pathology and genetics of tumors of the lung, pleura, thymus and heart. Lyon: IARC Press;2004. p. 45–50.
10. Haas JE, Palmer NF, Weinberg AG, Beckwith JB. Ultrastructure of malignant rhabdoid tumor of the kidney. A distinctive renal tumor of children. Hum Pathol. 1981; 12:646–657.
11. Park Y, Kim TS, Yi CA, Cho EY, Kim H, Choi YS. Pulmonary cavitary mass containing a mural nodule: differential diagnosis between intracavitary aspergilloma and cavitating lung cancer on contrast-enhanced computed tomography. Clin Radiol. 2007; 62:227–232.
12. Wu CY, Hu HY, Pu CY, Huang N, Shen HC, Li CP, et al. Pulmonary tuberculosis increases the risk of lung cancer: a population-based cohort study. Cancer. 2011; 117:618–624.
13. Hiroshima K, Shibuya K, Shimamura F, Toyozaki T, Haga Y, Ohwada H, et al. Pulmonary large cell carcinoma with rhabdoid phenotype. Ultrastruct Pathol. 2003; 27:55–59.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download