Abstract
Background
DNA damage-inducible 1 (Ddi1), one of the ubiquitin-like and ubiquitin-associated family of proteins, may function in the regulation of the ubiquitin-proteasome pathway, which has been validated as a target for antineoplastic therapy. We investigated Ddi1 expression in human lung cancer tissues and evaluated the relationship of this expression pattern with clinicopathological factors in patients with non-small-cell lung cancer (NSCLC).
Methods
Ddi1 expression was examined by immunohistochemistry in tumor tissues from 97 patients with stage I NSCLC, who had undergone curative surgical resection at two tertiary referral hospitals from 1993~2004. None of the patients received preoperative chemotherapy and/or radiation therapy.
Results
Thirty-nine (40.2%) of the 97 cases were positive for Ddi1. Ddi1 expression was dominantly seen in cytoplasm rather than in the nuclei of cancer cells in all histological types, whereas adjacent nontumoral lung tissue showed negative Ddi1 staining in most cases. Ddi1 expression tended to increase in well-differentiated tumors but without statistical significance. Positive Ddi1 expression was associated with a tendency for better disease-free survival and disease-specific survival, although the difference was not significant.
Lung cancer is one of the most commonly diagnosed cancers as well as the leading cause of cancer deaths worldwide1. However, only about 20% of patients with non-small cell lung cancer (NSCLC) have their disease diagnosed at a stage in which surgery can be recommended as part of initial care. Moreover, the 5-year survival rate of even the earliest stage I lung cancers after complete surgical resection is still only approximately 70%, leading to the need for novel therapeutic targets and modalities2.
One of the strategies for lung cancer therapy is targeting the ubiquitin-proteasome system (UPS), which plays a central role in cell homeostasis, resulting in deregulation of cell processes necessary for survival3,4. DNA damage-inducible 1 (Ddi1) belongs to the ubiquitin-like (UbL) and ubiquitin-associated (UBA) family of proteins that has been implicated in the regulation of UPS5. Ddi1 appears to release substrate from an ubiquitination complex, making the substrate available for deubiquitination, and, thus, is presumed to play an important role facilitating proteasomal degradation. Additionally, Ddi1 is the only representative in which the UbL and UBA domains flank an aspartyl protease-like (RVP) domain. This central Ddi1 domain has a remarkable structural similarity with retroviral proteases, suggesting that Ddi1 functions proteolytically during regulated protein turnover in the cell. Notably, current studies show that this RVP domain is a potential therapeutic target for retroviral aspartic protease inhibitors6.
Much effort has been made to reposition the established retroviral aspartic protease inhibitors as anticancer agents for patients with NSCLC. Recent studies have shown that nelfinavir, ritonavir, and saquinavir inhibit the growth of NSCLC and every cell type among 60 kinds of cancer cells7. Nelfinavir was the most effective of all protease inhibitors tested, resulting in apoptosis and non-apoptotic cell death. Non-apoptotic cell death was related to induction of endoplasmic reticulum stress, which subsequently led to autophagy, a normal process of self digestion that generates energy for the cell under conditions of stress8. Consequentially, these results suggest that nelfinavir could be repositioned as a lung cancer therapeutic, and one of the potential targets for this promising drug is Ddi1.
However, the clinical role of Ddi1 in human cancer is unknown. Therefore, in this study, we investigated Ddi1 immunohistochemical expression in human lung cancer tissue and evaluated whether Ddi1 expression increased in lung cancer compared with that in adjacent nontumoral tissue. We also investigated Ddi1 function by assessing intracellular localization as well as tissue distribution of Ddi1 expression to confirm whether Ddi1 could be developed as a useful target for nelfinavir.
The study group comprised 97 patients with stage I NSCLC. Patients underwent curative surgical resection at two tertiary referral hospitals (Severance and Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea) from 1993~2004. The follow-up duration was defined as the interval between the date of operation and the date of death or last follow-up. None of the patients received preoperative chemotherapy and/or radiation therapy.
Patient charts, including pathology and operative reports, were reviewed, and the data were coded. One representative formalin-fixed paraffin-embedded primary tumor block was obtained for each case.
This study was approved by the Institutional Review Board of Severance Hospital (4-2011-0409). The board waived informed consent from the patients. This study was conducted in accordance with the Declaration of Helsinki.
Sections (4-µm thick) were prepared from each of the paraffin-embedded samples. After sections were deparaffinized in xylene (three 10-minute washes) and dehydrated in a graded ethanol series (100-, 90-, 70-, and 50%, 5-minute washes), endogenous peroxidase activity was blocked with 30% hydrogen peroxide in water for 5 min. Antigens were retrieved for Ddi1 staining by boiling in 10 mM citrate acid (pH 6.0) for 3 min using a microwave oven. Sections were then incubated with rabbit anti-Ddi1 polyclonal antibodies (GeneTex, Irvine, CA, USA) and diluted 1:100 in antibody diluents (DAKO, Glostrup, Denmark) for 1 hour at 37℃. Secondary biotinylated link anti-mouse IgG or anti-rabbit IgG for the Labeled Streptavidin-Biotin2 System, horseradish peroxidase (DAKO), and a third streptavidin peroxidase conjugate (DAKO) were incubated with the sections at room temperature. The reaction was visualized with the 3, 3-diaminbenzidine substrate system (DAKO). Hematoxylin was used as the counterstain. Human placenta tissue was used as a positive control because of its easy availability and relatively stable reactivity. The negative control consisted of isotype control antibody (rabbit polyclonal IgG, DAKO) substituted for the primary Ddi1 antibody. Controls were run with each batch of slides at an average of approximately 15 slides per batch.
Slides were interpreted by one experienced pathologist (LBJ). The intensity of Ddi1 expression as evaluated microscopically was graded on a scale of 0~3+ with 3 being the highest expression observed (0, no staining; 1+, weak; 2+, moderate; 3+, intense). Frequency was quantified as the percentage of cells staining positive for Ddi1 with the primary antibody as follows: 0, no staining; 1, positive staining in <25 % of counted cancer cells; 2, positive staining in 25~50 % of counted cancer cells; 3, positive staining in >50 % of counted cancer cells. Intensity score (0~3+) was multiplied by the frequency score (0~3) to give an overall score of 0~9. The overall score for each specimen was then categorically assigned to one of the following groups: 0, negative expression; 1~2, weak expression; 3~6, moderate expression; 9, strong expression. Scores ≥1 were considered as positive.
Continuous variables were analyzed using nonparametric tests (Mann-Whitney U-test or Kruskal-Wallis test), and categorical variables were compared with the Pearson's χ2 or Fisher's exact tests, as appropriate. Survival curves were estimated using the Kaplan-Meier method, and statistical significance between survival curves was assessed by the log-rank test. Multivariate analysis was performed using Cox's proportional hazards regression model. SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. A p-value <0.05 was considered significant.
The demographic and clinical characteristics of the enrolled patients are listed in Table 1. Age, gender, and histological distributions in our population agreed with the literature for stage I NSCLC9,10. The median age of the patients was 64 years (range, 32~83 years), and the majority of patients were male (78.4%). The NSCLC tumors were comprised of 48 squamous cell carcinomas, 45 adenocarcinomas, three large-cell carcinomas, and one adenosquamous cell carcinoma. Twenty-three (23.7%) patients were stage IA and 74 (76.3%) were stage IB.
Figure 1 shows IHC staining patterns for the positive and negative controls. Ddi1 expression was seen in the cytoplasm of most syncytiotrophoblasts in normal placenta, which served as the positive control.
Of the 97 NSCLC cases, 39 (40.2%) were interpreted as Ddi1 positive, including 21 (43.8%) of 48 squamous cell carcinomas, 17 (37.8%) of 45 adenocarcinomas, and one of three large cell carcinomas (Table 1). Ddi1 expression was localized to the tumor cells, whereas bronchial epithelial cells, pneumocytes, and chondrocytes from bronchial cartilage in the adjacent nontumoral lung tissue showed Ddi1 negative staining in most cases examined. In all of the positive cases, Ddi1 expression was seen dominantly in the cytoplasm of cancer cells rather than in the nucleus, regardless of histological type. A cytoplasmic membrane staining pattern was observed in addition to the cytoplasmic and nuclear staining in one case of poorly differentiated adenocarcinoma (Figure 1).
Table 1 compares various parameters between the Ddi1 negative and positive groups. Histological subtype was not related to Ddi1 expression. Age, gender, smoking history, and pathological stage also did not differ between the groups. Ddi1 expression tended to increase with NSCLC differentiation; however, the difference was not significant. Table 2 shows Ddi1 expression according to histological subtype. No differences in age, pathological stage, or histological differentiation were observed between the Ddi1 expression groups when subgroup analyses were performed for the histological subtypes (Table 3).
The median follow-up duration was 120 months. In a univariate analysis, the positive Ddi1 expression group tended to show lower rates of tumor recurrence, distant metastasis, cancer-related death, and all-cause mortality but without statistical significance (Table 4).
Of 97 patients, 12 (30.8%) of 39 Ddi1 positive patients and 23 (39.7%) of 58 Ddi1 negative patients experienced a recurrence. The Kaplan-Meier disease-free survival (DFS) estimates for patients grouped according to Ddi1 expression are shown in Figure 2A; the median DFS time was 168.2 months in the Ddi1 positive group but was not reached in the Ddi1 negative group. Five-year DFS rate of Ddi1 positive group was 79.2% whereas that of Ddi1 negative group was 72.3%, which did not reach statistical significance (p=0.476; log-rank test).
Nine (23.1%) and 16 (27.6%) cancer-related deaths occurred in Ddi1 positive and negative patients, respectively. The median disease-specific survival (DSS) time was not reached in either Ddi1 expression group, whereas the estimated 5-year DSS rate was the same (81.4%) (Figure 2B).
The median overall survival (OS) times were 152.8 and 174.4 months, and the estimated 5-year OS rates were 73.0% and 74.1% in the Ddi1 positive and negative groups. The Kaplan-Meier estimates for both groups showed no significant difference in OS (Figure 2C). The Cox regression analysis adjusting for pathological stage and age demonstrated no significant difference in DFS, DSS, or OS between the Ddi1 expression groups (data not shown).
The results were similar when subgroup analyses were performed for the histological subtypes. Ddi1 expression did not significantly influence DFS, DSS, or OS of patients with squamous cell carcinoma or adenocarcinoma (data not shown).
Among the UbL-UBA family of proteins, Ddi1 is the least studied and has never been associated with lung cancer. In the present study, IHC showed positive Ddi1 staining in about 40% of lung cancer tissues, and the proportions were similar among histological subtypes. Among the positive cases, most of the adjacent nontumoral lung tissue was negative for Ddi1 expression, whereas cancer cells showed diffuse staining for Ddi1, suggesting that Ddi1 could be a potential selective target for lung cancer treatment.
Previous studies have shown that Ddi1 localizes preferentially to the nucleus in wild-type cells, which could imply its function in nuclear-associated ubiquitin-dependent proteolysis11,12. Unexpectedly, we found that Ddi1 expression occurred not only in the nucleus, but also in the cytoplasm, even in the cell membranes of cancer cells. Therefore, we postulate that any alteration of the role of Ddi1 in the interaction with cell cycle-related proteins and mediating their stability may have influenced Ddi1 localization in NSCLC cells.
The role of Ddi1 in targeting proteins for proteasomal degradation is well established. A loss of Ddi1 results in stabilization of ubiquitinated substrates, which accumulate in the cytoplasm. The substrate can only interact with the proteasome in the presence of Ddi1 when it is ubiquitinated, suggesting that the initial interaction must be between a ubiquitin chain and the Ddi1 protein11. Because ubiquitination is critical for proliferation and survival of cancer cells, Ddi1 dysregulation may result in cancer by creating a UPS imbalance.
In the present study, poor differentiation of tumor cells tended to be correlated with negative Ddi1 expression, but the correlation was not significant. Different results have been reported on the relationship between tumor differentiation and expression of UPP components. Some studies have reported that cancers and rapidly growing embryonic cells generally have higher levels of proteasome activity than their normal well-differentiated counterparts13,14, and others have found that Ub levels increase during development and differentiation15,16. In contrast, another study showed no relationship between ubiquitin levels and the degree of histological differentiation17. The present results showed that positive Ddi1 expression appeared to be associated with well-differentiated tumors, which behave in an indolent fashion. Thus, we presumed that Ddi1 might play a greater role during the initial stage of carcinogenesis than in the progressing stage when tumors develop more aggressive behavior, although it is difficult to assume the common roles of Ddi1 during oncogenesis. This possibility parallels our results showing a higher rate of local recurrence than distant metastasis in recurred Ddi1 positive patients, whereas a higher rate of distant metastasis was observed in patients who were Ddi1 negative. Furthermore, Ddi1 expression seemed to be associated with a more indolent clinical course with better disease-free and disease-specific survivals. However, these results did not reach statistical significance, probably because of the relatively small sample size; thus, further studies are needed to confirm or refute this association.
In conclusion, we observed positive Ddi1 staining in about 40% of NSCLC tissues. Our results suggest that Ddi1 expression is a property of NSCLC, though it is not definite whether Ddi1 has clinical value in NSCLC. Ddi1 could be a potential target for cancer therapy. Therefore, more work is needed to evaluate the role of Ddi1 in NSCLC.
Figures and Tables
Figure 1
DNA damage-inducible 1 (Ddi1) expression in controls and lung cancer tissues; (A) positive control, (B) negative control, (C) bronchioalveolar carcinoma, (D) adenocarcinoma, (E) squamous cell carcinoma, (F) large cell carcinoma, and (G) adjacent nontumoral lung tissue (×200).
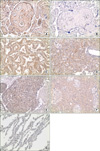
Figure 2
Survival analysis of patients with stage I non-small-cell lung cancer stratified for DNA damage-inducible 1 (Ddi1) expression; (A) disease free survival, (B) disease specific survival and (C) overall survival of patients with non-small-cell lung cancer.

Table 1
Clinicopathological features related to DNA damage-inducible 1 (Ddi1) expression in non-small-cell lung cancer (NSCLC)

Table 2
DNA damage-inducible 1 (Ddi1) expression according to non-small-cell lung cancer (NSCLC) histological subtype
References
1. Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest. 2002. 122:1037–1057.
2. Groome PA, Bolejack V, Crowley JJ, Kennedy C, Krasnik M, Sobin LH, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007. 2:694–705.
3. Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994. 79:13–21.
4. Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. 2009. 458:438–444.
5. Su V, Lau AF. Ubiquitin-like and ubiquitin-associated domain proteins: significance in proteasomal degradation. Cell Mol Life Sci. 2009. 66:2819–2833.
6. White RE, Powell DJ, Berry C. HIV proteinase inhibitors target the Ddi1-like protein of Leishmania parasites. FASEB J. 2011. 25:1729–1736.
7. Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, Abu-Asab MS, et al. Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res. 2007. 13:5183–5194.
8. Gills JJ, Lopiccolo J, Dennis PA. Nelfinavir, a new anti-cancer drug with pleiotropic effects and many paths to autophagy. Autophagy. 2008. 4:107–109.
9. Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients: a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer. 2007. 110:1532–1541.
10. Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P, et al. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009. 4:792–801.
11. Kaplun L, Tzirkin R, Bakhrat A, Shabek N, Ivantsiv Y, Raveh D. The DNA damage-inducible UbL-UbA protein Ddi1 participates in Mec1-mediated degradation of Ho endonuclease. Mol Cell Biol. 2005. 25:5355–5362.
12. Wilkinson CR, Wallace M, Morphew M, Perry P, Allshire R, Javerzat JP, et al. Localization of the 26S proteasome during mitosis and meiosis in fission yeast. EMBO J. 1998. 17:6465–6476.
13. Kumatori A, Tanaka K, Inamura N, Sone S, Ogura T, Matsumoto T, et al. Abnormally high expression of proteasomes in human leukemic cells. Proc Natl Acad Sci USA. 1990. 87:7071–7075.
14. Kanayama H, Tanaka K, Aki M, Kagawa S, Miyaji H, Satoh M, et al. Changes in expressions of proteasome and ubiquitin genes in human renal cancer cells. Cancer Res. 1991. 51:6677–6685.
15. Goldknopf IL, Wilson G, Ballal NR, Busch H. Chromatin conjugate protein A24 is cleaved and ubiquitin is lost during chicken erythropoiesis. J Biol Chem. 1980. 255:10555–10558.
16. Agell N, Mezquita C. Cellular content of ubiquitin and formation of ubiquitin conjugates during chicken spermatogenesis. Biochem J. 1988. 250:883–889.
17. Ishibashi Y, Takada K, Joh K, Ohkawa K, Aoki T, Matsuda M. Ubiquitin immunoreactivity in human malignant tumours. Br J Cancer. 1991. 63:320–322.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download