Abstract
Bronchopulmonary sequestration (BPS) is a rare congenital malformation of the lower respiratory tract. Most intralobar BPSs are provided with an arterial blood via the thoracic or abdominal aorta but such a supply is rarely found in patients older than 50 years. We report a case of an intralobar BPS with a dual arterial supply from the celiac artery and thoracic aorta in a 50-year-old man presenting with a respiratory tract infection and haemoptysis. To our knowledge, this is the first case report of a BPS supplied by the celiac artery and thoracic aorta in a 50-year-old man.
Brochopulmonary sequestration (BPS) is a rare abnormality among the spectrum of congenital bronchopulmonary malformations. It is characterized by a mass of nonfunctioning lung tissues, which is supplied with arterial blood by a systemic artery and is separated from the normal tracheobronchial tree1. BPS is subdivided into two types based on whether or not abnormal lung tissues possess their own pleural covering. Intralobar BPS does not have their own pleural covering, as they are located on a normal lobe and share pleura with the normal lung tissues. Extralobar BPS has a separate lining of pleura, because they are located outside the normal lung2-5. Intralobar sequestration is typically diagnosed in pediatric or adolescent patients, with equal gender prevalence. These are rarely found in patients older than 50 years6. Most intralobar BPSs are supplied with arterial blood by the thoracic or abdominal aorta2. We report a case of BPS with dual arterial supply by the celiac artery and thoracic aorta in 50-years old patient who was diagnosed by three dimensional computed tomographic (3-D CT) angiography and treated successfully by a surgical resection
A 50-year-old man was admitted to our hospital with intermittent cough, and hemoptysis that had begun one week before the admission in December, 2008. He was a smoker and heavy drinker who consumed 30 packs of cigarettes a year and four to five bottles of Korean distilled liquor, a week. He had been diagnosed with and treated for pneumonia, which had been caused by bronchiectasis and recurred once or twice a year, at another hospital several years ago. His occupation was farming.
He denied any allergic history and adverse drug reaction. His parents and siblings were clinically healthy.
Physical examination showed his blood pressure at 130/80 mm Hg, pulse rate of 88/min, respiratory rate of 20/min and 36.8℃ body temperature. Auscultation detected crackles in the right lower lung field.
The laboratory findings on admission were as follow: white blood cell counts 11,400/µL, hemoglobin 14.8 g/dL, platelet counts 286,000/µL, aspartate aminotransferase 12 IU/L, alanine aminotransferase 5 IU/L, total bilirubin 0.9 mg/dL, blood urea nitrogen 12 mg/dL, Creatinine 0.8 mg/dL, and C reactive protein 6.69 mg/dL.
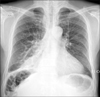
Chest PA revealed dense consolidation blurring the right cardiac border of the right lower lung field, costophrenic angle blunting in the right, and increased pulmonary vascularities (Figure 1). The computed tomographic (CT) scan with contrast enhancement of the chest showed an 8.0×7.8 cm mass in the right middle lobe, mainly. The mass was composed of nodular soft tissues and filled with fluid. Air fluid level in the cysts of the right lower lobe suggested bronchiectasis (Figure 2).
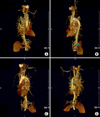
3-D CT angiography showed a large meandering abnormal artery originated from the celiac artery and another small meandering abnormal artery from the thoracic aorta. These two arteries fed to an area on the right middle and lower lung fields (Figure 3). The CT scan and 3-D CT angiography indicated intralobar BPS. In December 2008, he was referred to other hospital in Seoul for a surgical resection.
Thoracotomy and bilobectomy were carried out on the right middle and lower lobe. After bilobectomy, he was clinically well during a six-month follow up.
BPS is a rare congenital anomaly that accounts for 0.15~6.4% of congenital pulmonary malformations2 and 1.1~1.8% of pulmonary resections3. Among the two types of BPS, intralobar BPS is more common, representing approximately 75~90% of BPS2,7.
Intralobar BPS is diagnosed in the second decade of life or younger in approximately 50~60% of cases, and rarely found in patients older than 50 years6. As the patient reported here, most senile patients experience chronic cough, sputum, and recurrent attacks of pneumonia similarly with juvenile patients8. Infection-associated hemoptysis is also a common presenting sign among elderly patients8. After reviewing 373 patients with intralobar BPS, Savic et al2 reported that the arterial supply to intralobar BPS originated from the descending thoracic aorta in 73.9% of the cases, the abdominal aorta in 18.7% and the intercostals arteries in 3.2%. Another study also reported that arterial supply is predominantly driven from the descending thoracic aorta of the left lung. The most common location for aberrant artery was the basal segment of the left lower lobe among juvenile patients9 and the descending thoracic aorta among senile patients (nine patients or 90%). All lesions were observed in the left lower lobe, mostly commonly at its basal segment8. In contrast to these studies, however, the lesion reported here was located in the right middle and lower lobe with aberrant arteries arising from the celiac artery and thoracic aorta. It is the first reported case of intralobar BPS with dual arterial supply from the celiac artery and thoracic aorta in 50-year-old man.
Last but not least, angiography is required for definitive diagnosis of BPS. However, it was not performed on the patient here, because 3-D CT angiography provided accurate information on the aberrant vascular supply.
Figures and Tables
Figure 1
Chest PA showed dense consolidation blurring the right cardiac border of the right lower lung field.
References
1. Landing BH, Dixon LG. Congenital malformations and genetic disorders of the respiratory tract (larynx, trachea, bronchi, and lungs). Am Rev Respir Dis. 1979. 120:151–185.
2. Savic B, Birtel FJ, Tholen W, Funke HD, Knoche R. Lung sequestration: report of seven cases and review of 540 published cases. Thorax. 1979. 34:96–101.
3. Carter R. Pulmonary sequestration. Ann Thorac Surg. 1969. 7:68–88.
4. Sade RM, Clouse M, Ellis FH. The spectrum of pulmonary sequestration. Ann Thorac Surg. 1974. 18:644–658.
5. O'Mara CS, Baker RR, Jeyasingham K. Pulmonary sequestration. Surg Gynecol Obstet. 1978. 147:609–616.
6. Petersen G, Martin U, Singhal A, Criner GJ. Intralobar sequestration in the middle-aged and elderly adult: recognition and radiographic evaluation. J Thorac Cardiovasc Surg. 2003. 126:2086–2090.
7. Clements BS. Taussig LM, Landa LI, editors. Congenital malformations of the lungs and airways. Pediatric Respiratory Medicine. 1999. St. Louis: Mosby;1106.
8. Hirai S, Hamanaka Y, Mitsui N, Uegami S, Matsuura Y. Surgical treatment of infected intralobar pulmonary sequestration: a collective review of patients older than 50 years reported in the literature. Ann Thorac Cardiovasc Surg. 2007. 13:331–334.
9. Yamanaka A, Hirai T, Fujimoto T, Hase M, Noguchi M, Konishi F. Anomalous systemic arterial supply to normal basal segments of the left lower lobe. Ann Thorac Surg. 1999. 68:332–338.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download