A variety of abdominal symptoms such as nausea, vomiting and abdominal distension caused by intestinal obstruction can develop in patients with lung cancer. Patients often become frustrated and their medical condition tend to debilitate because prolonged naso-gastric tube insertion and parenteral nutrition through venous catheter is required regardless of whether intestinal obstruction is functional or mechanical. This may be related to treatment or the disease itself. Prompt and proper discovery of cause is important because approach for treatment may differ according to its etiology and emergency operation can often be required to prevent more severe complications1,2, although this may not be easy even with various diagnostic technologies. Therefore, physicians need to be aware of the possible etiologies which may lead to intestinal obstruction in lung cancer. Herein, we report two cases presenting with symptoms associated with intestinal obstruction from different causes.
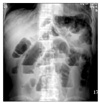
A 57-year-old man was transferred from a local hospital for evaluation of nausea and bilious vomiting which developed during radiotherapy to the cervical spine. He was diagnosed to have non-small-cell lung cancer with multiple metastases to the brain, lung and bones one month prior to admission. Previously, the patient had received palliative radiotherapy of 3,000 cGy over two weeks to the brain and subsequent radiotherapy to the cervical spine for palliation of neck pain. The patient's body weight was 52 kg and height was 172 cm. He had lost 10 kg over two months due to severe anorexia. After insertion of naso-gastric tube, symptoms were relieved. However, symptoms of nausea and bilious vomiting were repeatedly aggravated whenever the naso-gastric tube was removed which required reinsertion of the tube. An approximate 7 cm-sized mass in the RLL with multiple metastatic nodules was noted on simple chest radiograph and Chest CT. Review of biopsied specimen confirmed the diagnosis of non-small-cell lung cancer. Round gaseous bowel distensions in central part of the abdomen were found on simple abdominal radiograph (Figure 1A) while no definite mass was visible on abdominal CT. However, abdominal CT showed that the dilatation was limited to the stomach, duodenal bulb and 2nd portion raising the suspicion of superior mesenteric artery (SMA) syndrome (Figure 1B). To confirm this, upper gastrointestinal series was performed, which also demonstrated passage disturbance of contrast dye to the duodenal 3rd portion (Figure 1C). Surgical intervention could not be considered due to the patient's poor general condition. His condition continuously deteriorated after development of pneumonia and finally he succumbed to death.
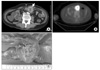
A 68-year-old man was admitted for abdominal pain, nausea and vomiting of 2 weeks duration. He had undergone surgery and adjuvant chemotherapy for non-small-cell lung cancer 3 years ago. The post-operative pathologic stage was T2N2M0, IIIA. Two years later, a recurrent mass just distal to the bronchial stump with mediastinal and abdominal lymph node enlargement was found. Despite palliative chemotherapy with gemcitabine plus carboplatin and pemetrexed, his disease continuously progressed over one year. On admission, typical step-ladder appearance suggestive of mechanical ileus was detected on simple abdominal radiograph (Figure 2). Abdominal CT showed a round mass in the abdominal cavity near the transverse colon (Figure 3A). The mass was hypermetabolic on PET/CT with a SUV of 7.7 (Figure 3B). In spite of progressive disease, the patients general condition was not so poor, hence palliative operation was done. The 4 cm-sized mass was found to be located on the jejunal wall (Figure 3C) and segmental small bowel resection was performed. Unfortunately, severe pneumonia developed four months after surgery and finally he succumbed to death.
In fact, symptomatic small bowel metastases from lung cancer have been rarely reported although gastrointestinal metastasis from lung cancer is not uncommon in autopsy series3,4 and small bowel is the most common site of metastasis within the gastrointestinal tract1,2. In previous series, the incidence of symptomatic small bowel metastases of lung cancer is about 0.1~0.6% and there were several small bowel obstruction cases ever reported1,2. Nevertheless, if lung cancer patients without possible etiologies to cause intestinal obstruction such as previous history of abdominal operation present with symptoms suggesting intestinal obstruction, small bowel metastasis should be considered because surgical intervention is often required to relieve obstruction or prevent life-threatening complications such as perforation and hemorrhage5,6. In our case, the use of 18FDG-PET/CT has made the diagnosis of intestinal metastasis easier than in the past1.
SMA syndrome develops by extrinsic compression of the 3rd portion of the duodenum between the SMA and the aorta. Several conditions causing rapid and marked weight loss such as prolonged immobilization from trauma or burn7, malabsorption, and anorexia nervosa8 can result in this syndrome although anatomical anomalies9 and surgical complications10,11 can also be the cause. In our case, rapid weight loss over 2 months from severe anorexia and emesis may have lead to loss of mesenteric and retroperitoneal fat subsequently resulting in decrease of the aortomesenteric angle. Although there have been some reports of SMA syndrome related with malignancy, most of them occurred as complications after operation or radiotherapy12,13. To the best of our knowledge, this is the first reported case of SMA syndrome in lung cancer.
Patients with SMA syndrome usually complain of non-specific symptoms including nausea, vomiting, epigastric pain and bloating. However, history of bilious and voluminous vomiting which mostly occurs shortly after meals and is often relieved by postural changes may help with the diagnosis14,15. In many cases, diagnosis is made by contrast medium swallow with follow-through imaging showing an abrupt cut-off of barium in the distal duodenum and delayed gastric emptying16. It can also be made by contrast-enhanced spiral CT or MR angiography. Endoscopy may help to exclude other obstructing abnormalities within the upper gastrointestinal tract. Although endoscopy itself has little diagnostic value to confirm this syndrome, combination with ultrasound can visualize the pulsating nature of duodenal compression and the reduced aortomesenteric distance15. Because it is very rare and its diagnosis is frequently made by exclusion, this syndrome can sometimes be a diagnostic challenge to physicians. Therefore, awareness of this rare entity and careful history taking are essential for proper diagnosis.
Surgery is recommended if conservative management including total parenteral hyperalimentation or enteral feeding past the ligament of Treitz to restore retroperitoneal fat fails. Duodenojejunostomy has been preferred10 although alternative procedures such as division of the ligament of Treitz17 or the Ladd method of mobilization and derotation of the duodenum and colon11 have been suggested.
Physicians should be aware of possible etiologies resulting in intestinal obstruction in lung cancer patients to provide early diagnosis and appropriate management.
Prompt and proper discovery of cause is important in lung cancer patients with signs and symptoms of intestinal obstruction because approach for treatment may differ according to its etiology and emergency operation can often be required to prevent more severe complications. In this report, we present two different causes of intestinal obstruction in lung cancer. Physicians need to be aware of these possibilities to differentiate the cause of intestinal obstruction in patients with lung cancer.
Figures and Tables
Figure 1
(A) Plain abdominal radiograph shows gaseous distension of the stomach, the duodenal bulb, and 2nd portion. (B) On the enhanced CT scan obtained at the level of the duodenal 3rd portion, the duodenum coursing between the superior mesenteric artery and the aorta is compressed by the vascular structures leading to dilatation of the proximal part. (C) Double contrast barium study of the upper gastrointestinal tract demonstrates the luminal narrowing of the duodenal 3rd portion corresponding to the site between the superior mesenteric artery and the aorta.

References
1. Kim MS, Kook EH, Ahn SH, Jeon SY, Yoon JH, Han MS, et al. Gastrointestinal metastasis of lung cancer with special emphasis on a long-term survivor after operation. J Cancer Res Clin Oncol. 2009. 135:297–301.
2. Yang CJ, Hwang JJ, Kang WY, Chong IW, Wang TH, Sheu CC, et al. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer. 2006. 54:319–323.
3. Antler AS, Ough Y, Pitchumoni CS, Davidian M, Thelmo W. Gastrointestinal metastases from malignant tumors of the lung. Cancer. 1982. 49:170–172.
4. McNeill PM, Wagman LD, Neifeld JP. Small bowel metastases from primary carcinoma of the lung. Cancer. 1987. 59:1486–1489.
5. Yoshimoto A, Kasahara K, Kawashima A. Gastrointestinal metastases from primary lung cancer. Eur J Cancer. 2006. 42:3157–3160.
6. Ise N, Kotanagi H, Morii M, Yasui O, Ito M, Koyama K, et al. Small bowel perforation caused by metastasis from an extra-abdominal malignancy: report of three cases. Surg Today. 2001. 31:358–362.
7. Milner EA, Cioffi WG, McManus WF, Pruitt BA Jr. Superior mesenteric artery syndrome in a burn patient. Nutr Clin Pract. 1993. 8:264–266.
8. Pentlow BD, Dent RG. Acute vascular compression of the duodenum in anorexia nervosa. Br J Surg. 1981. 68:665–666.
9. Coster DD, Stubbs DH, Sidney DT. Duodenal obstruction by abdominal aortic aneurysms. Am J Gastroenterol. 1988. 83:981–984.
10. Derincek A, Wood KB, Muench CA. Superior mesenteric artery syndrome following correction of kyphosis in an adult. J Spinal Disord Tech. 2004. 17:549–553.
11. Ballantyne GH, Graham SM, Hammers L, Modlin IM. Superior mesenteric artery syndrome following ileal J-pouch anal anastomosis. An iatrogenic cause of early postoperative obstruction. Dis Colon Rectum. 1987. 30:472–474.
12. Boldery J, Gleeson J, Jordaan J. Superior mesenteric artery syndrome following small bowel resection. ANZ J Surg. 2006. 76:861–862.
13. Klee FE, Osswald BR, Wysocki S. Testicular tumors, abdominal radiotherapy, and superior mesenteric artery syndrome. J Clin Oncol. 1993. 11:1626–1627.
14. Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome. Diagnostic criteria and therapeutic approaches. Am J Surg. 1984. 148:630–632.
15. Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol. 2002. 37:640–643.
16. Griffiths GJ, Whitehouse GH. Radiological features of vascular compression of the duodenum occurring as a complication of the treatment of scoliosis (the cast syndrome). Clin Radiol. 1978. 29:77–83.
17. Massoud WZ. Laparoscopic management of superior mesenteric artery syndrome. Int Surg. 1995. 80:322–327.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download