Abstract
Background
The present investigation was performed in rats and isolated human neutrophils in order to confirm the presumptive role of the positive feedback loop of cytosolic phospholipase A2 (cPLA2) activation by platelet-activating factor (PAF).
Methods
The possible formation of the positive feedback loop of the cPLA2 activation and neutrophilic respiratory burst was investigated in vivo and in vitro by measurement of the parameters denoting acute lung injury. In addition, morphological examinations and electron microscopic cytochemistry were performed for the detection of free radicals in the lung.
Results
Five hours after intratracheal instillation of PAF (5µg/rat), the lung leak index, lung myeloperoxidase (MPO) activity, the number of neutrophils and the concentration of cytokine-induced neutrophil chemoattractant (CINC) in bronchoalveolar lavage fluid were increased by PAF as compared with those of control rats. The NBT assay and cytochrome-c reduction assay revealed an increased neutrophilic respiratory burst in isolated human neutrophils following exposure to PAF. Lung and neutrophilic cPLA2 activity were increased following PAF exposure and exposure to hydrogen peroxide increased cPLA2 activity in the lung. Histologically, inflammatory findings of the lung were observed after PAF treatment. Remarkably, as determined by CeCl3 cytochemical electron microscopy, increased production of hydrogen peroxide was identified in the lung after PAF treatment.
Figures and Tables
Figure 1
Histological changes in the lung after instillation of PAF (5µg) into the trachea. Perivascular cuffing (asterik, A), interstitial edema and infiltration of inflammatory cells were found (A, B). Accumulation of inflammatory cells in vascular lumen, interstitium and alveoli were found (arrow head, C, D). ×200.
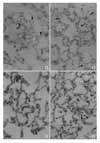
Figure 2
Electron microscopic finding of normal lung (A). Lamellar bodies, endothelial cells and type I alveolar cells were well preserved. In CeCl3 electron microscopic cytochemistry (B), deposits of cerrous perhydroxide were not found indicating oxidants were not generated in the normal lung. Bar indicates 2.5µm.
LB: lamellar body; En: endothelial cell; AL: alveolar lumen.

Figure 3
Ultrastructural changes in the lung of the rat given PAF (5µg) intratracheally. In routine electron microscopic finding (A), the infiltration of neutrophils in interstitium (NP), degeneration of lamellar bodies (arrow head), vacuolization of lamellar bodies (asterik) were noted. NP: neutrophil. Bar indicates 2.0µm. In CeCl3 cytochemical electron microscopy, deposits of cerrous perhydroxide granules were indentified in the interstitium and to the proximity of neutrophils (B). NP: neutrophil; Bar indicates 1.0µm.

Table 1
Comparisons of lung leak index, lung MPO, BAL PMNLs, CINC concentration between sham and PAF-treated rats

Table 2
Comparison of indeces of neutrophilic oxidative stress between sham and PAF-treated neutrophils

References
1. Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006. 1761:1246–1259.
2. Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997. 155:1187–1205.
3. Pruzanski W, Vadas P. Phospholipase A2-a mediator between proximal and distal effectors of inflammation. Immunol Today. 1991. 12:143–146.
4. Harris JG, Flower RJ, Watanabe K, Tsurufuji S, Wolitzky BA, Perretti M. Relative contribution of the selections in the neutrophil recruitment caused by the chemokine cytokine induced neutrophil chemoattractant. Biochem Biophys Res Commun. 1996. 221:692–696.
5. Schuster DP, Kollef MH. Acute respiratory distress syndrome. Dis Mon. 1996. 42:270–326.
6. Henderson LM, Chappell JB, Jones OT. Superoxide generation is inhibited by phospholipase A2 inhibitors. Role for phospholipase A2 in the activation of the NADPH oxidase. Biochem J. 1989. 264:249–255.
7. Dana R, Leto TL, Malech HL, Levy R. Essential requirement of cytosolic phospholipase A2 for activation of the phagocyte NADPH oxidase. J Biol Chem. 1998. 273:441–445.
8. Rainger GE, Fisher AC, Nash GB. Endothelial-borne platelet-activating factor and interleukin-8 rapidly immobilize rolling neutrophils. Am J Physiol. 1997. 272:H114–H122.
9. Uhlig S, Goggel R, Engel S. Mechanisms of platelet-activating factor (PAF)-mediated responses in the lung. Pharmacol Rep. 2005. 57S:206–221.
10. Takahashi T, Nishizawa Y, Hato F, Shintaku H, Maeda N, Inaba M, et al. Neutrophil-activating activity and platelet-activating factor synthesis in cytokine-stimulated endothelial cells: reduced activity in growth-arrested cells. Microvasc Res. 2007. 73:29–34.
11. Lee YM, Hybertson BM, Cho HG, Terada LS, Cho O, Repine AJ, et al. Platelet-activating factor contributes to acute lung leak in rats given interleukin-1 intratracheally. Am J Physiol Lung Cell Mol Physiol. 2000. 279:L75–L80.
12. Camussi G, Pawlowski I, Tetta C, Roffinello C, Alberton M, Brentjens J, et al. Acute lung inflammation induced in the rabbit by local instillation of 1-0-octadecyl-2-acetyl-sn-glyceryl-3-phosphorylcholine or of native platelet-activating factor. Am J Pathol. 1983. 112:78–88.
13. Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol. 1985. 59:1978–1985.
14. Wittwer AJ, Carr LS, Zagorski J, Dolecki GJ, Crippes BA, De Larco JE. High level expression of cytokine-induced neutrophil chemoattractant (CINC) by a metastatic rat cell line: purification and production of blocking antibodies. J Cell Physiol. 1993. 156:421–427.
15. Prasad K, Kapoor R, Lee P. Purpurogallin, a scavenger of polymorphonuclear leukocyte-derived oxyradicals. Mol Cell Biochem. 1994. 139:27–32.
16. Haslett C, Guthrie LA, Kopaniak MM, Johnston RB Jr, Henson PM. Modulation of multiple neutrophil function by preparative methods of trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985. 119:101–110.
17. Botha AJ, Moore FA, Moore EE, Fontes B, Banerjee A, Peterson VM. Postinjury neutrophil priming and activation states: therapeutic challenges. Shock. 1995. 3:157–166.
18. Katsumata M, Gupta C, Goldman AS. Rapid assay for activity of phospholipase A2 using radioactive substrate. Anal Boichem. 1986. 154:676–681.
19. Hobson J, Wright J, Churg A. Histochemical evidence for generation of active oxygen species on the apical surface of cigarette-smoke- exposed tracheal explants. Am J Pathol. 1991. 139:573–580.
20. Nakos G, Kitsiouli E, Hatzidaki E, Koulouras V, Touqui L, Lekka ME. Phospholipases A2 and platelet-activating-factor acetylhydrolase in patients with acute respiratory distress syndrome. Crit Care Med. 2005. 33:772–779.
21. Schuster DP, Metzler M, Opal S, Lowry S, Balk R, Abraham E, et al. Pafase ARDS Prevention Study Group. Recombinant platelet-activating factor acetylhydrolase to prevent acute respiratory distress syndrome and mortality in severe sepsis: Phase IIb, multicenter, randomized, placebo controlled, clinical trial. Crit Care Med. 2003. 31:1612–1619.
22. Prescott SM, McIntyre TM, Zimmerman G. Two of the usual suspects, platelet-activating factor and its receptor, implicated in acute lung injury. J Clin Invest. 1999. 104:1019–1020.
23. Koh Y, Hybertson BM, Jepson EK, Cho OJ, Repine JE. Cytokine-induced neutrophil chemoattractant is necessary for interleukin-1 induced lung leak in rats. J Appl Physiol. 1985. 79:472–478.
24. Suzuki YJ, Forman HJ, Sevenian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997. 22:269–285.
25. Repine JE. Scientific perspectives on adult respiratory distress syndrome. Lancet. 1992. 339:466–469.
26. Mantovani A, Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989. 10:370–375.
27. Serizawa A, Nakamura S, Suzuki S, Baba S, Nakano M. Involvement of platelet activating factor in cytokine production and neutrophil activation after hepatic ischemia-reperfusion. Hepatology. 1996. 23:1656–1663.
28. Tamm M, Bihl M, Eickelberg O, Stulz P, Perruchoud AP, Roth M. Hypoxia-induced interleukin-6 and interleukin-8 production is mediated by platelet-activating factor and platelet-derived growth factor in primary human lung cells. Am J Respir Cell Mol Biol. 1998. 19:653–661.
29. Anderson BO, Brown JM, Harken AH. Mechanisms of neutrophil-mediated tissue injury. J Surg Res. 1991. 51:170–179.
30. Lee YM, Hybertson BM, Terada LS, Repine AJ, Cho HG, Repine JE. Mepacrine decreases lung leak in rats given interleukin-1 intratracheally. Am J Respir Crit Care Med. 1997. 155:1624–1628.
31. Hybertson BM, Lee YM, Cho HG, Cho OJ, Repine JE. Alveolar type II cell abnormalities and peroxide formation in lungs of rats given IL-1 intratracheally. Inflammation. 2000. 24:289–303.
32. Martensson J, Jain A, Frayer W, Meister A. Glutathione metabolism in the lung: inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc Natl Acad Sci U S A. 1989. 86:5296–5300.
33. Kim FJ, Moore EE, Moore FA, Biffl WL, Fontes B, Banerjee A. Reperfused gut elaborates PAF that chemoattracts and primes neutrophils. J Surg Res. 1995. 58:636–640.
34. Balsinde J, Dennis EA. Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J Biol Chem. 1996. 271:6758–6765.
35. Nakashima S, Suganuma A, Sato M, Tohmatsu T, Nazawa Y. Mechanism of arachidonic acid liberation in platelet-activating factor-stimulated human polymorphonuclear neutrophils. J Immunol. 1989. 143:1295–1302.
36. Sun X, Caplan MS, Hsueh W. Tumor necrosis factor and endotoxin synergistically activate intestinal phospholipase A2 in mice. Role of endogenous platelet activating factor and effect of exogenous platelet activating factor. Gut. 1994. 35:215–219.
37. Boyer CS, Bannenberg GL, Neve EP, Ryrfeldt A, Moldeus P. Evidence for the activation of the signal-responsive phospholipase A2 by exogenous hydrogen-peroxide. Biochem Pharmacol. 1995. 50:735–761.
38. Chakraborti S, Michael JR. Role of protein kinase C in oxidant-mediated activation of phospholipase A2 in rabbit pulmonary arterial smooth muscle cells. Mol Cell Biochem. 1993. 122:9–15.
39. Lee YM, Hybertson BM, Cho HG, Repine JE. Platelet activating factor (PAF) induces lung inflammation and leak in rats: hydrogen peroxide production along neutrophil-lung endothelial interfaces. J Lab Clin Med. 2002. 140:312–319.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download