Abstract
The major risk factor for ovarian cancer (OC) is mutation of the BRCA1 or BRCA2 DNA mismatch repair genes, which occurs in approximately 10% of OC cases. Most previous studies have demonstrated that BRCA1- and BRCA2-mutated OCs are associated with better prognosis than sporadic OCs. However, information about the patterns and clinical course of the metastatic spread of BRCA-mutated OCs is limited. Herein, we describe a case of OC with a BRCA1 mutation and skin metastases in a 49-year-old patient, which to the best of our knowledge has not been reported previously.
BRCA1 and BRCA2 mutations are found in families with multiple cases of cancer that include a high proportion of ovarian cancers (OCs) and, most notably, at least one case of breast cancer [1]. Previously accumulated data have shown a substantial proportion of inter-individual heterogeneity in genetic predisposition associated with OC risk, and therefore, screening for mutations in the OC susceptibility genes BRCA1 and BRCA2 has emerged as a predictive genetic testing for the assessment of lifetime risk of OC.
BRCA mutations are present in more than 10% of OC cases. Women who had hereditary BRCA-mutated OC had a better prognosis compared with women who had sporadic OC [23]. Cass et al. [4] demonstrated that BRCA-mutated OC might be associated with an improved response to chemotherapy; this chemosensitivity in patients with BRCA-mutated OC may result from an impaired ability of BRCA-deficient cells to repair DNA damaged by chemotherapy-induced cytotoxicity [5]. However, despite the favorable survival outcome of BRCA-mutated OCs reported in previous studies, there is currently no data regarding the practical course of OC with BRCA mutation, such as patterns of metastatic spread or sites of distant recurrence.
The clinical course of OC spreads early in its development, and more than 60% of women, at the time of diagnosis, have stage III or stage IV OC, indicating metastasis beyond the ovaries has already occurred. Several studies reported the prevalence of distant metastases in advanced stages of epithelial OC. For example, Dauplat et al. [6] showed that pleural metastases were the most common occurrence (24.7%), followed by metastatic lesions in the liver, lungs, and lymph nodes; cutaneous metastases had developed in only 3.5% of the patients with OC. In another study, the incidence of skin metastases ranged between 1.9% and 5.1% [7]. However, so far, skin metastases in BRCA-mutated OCs have not been reported. Therefore, herein, we report an unusual presentation of cutaneous metastasis from an OC with a BRCA1 mutation, which to the best of our knowledge, has not been reported previously.
A previously healthy 49-year-old woman with a previous diagnosis of stage IIIC ovarian seromucinous adenocarcinoma was receiving fourth-line chemotherapy (cisplatin, doxorubicin, and cyclophosphamide every 3 weeks) owing to newly detected metastases in both the adrenal glands and the left psoas muscle. She was gravida 1, para 1, with a prior normal vaginal delivery of a healthy, full-term male infant. Although she had no obvious family history that suggested hereditary OC susceptibility, she was offered BRCA testing for familial risk assessment. DNA was extracted and purified from peripheral blood samples, and aliquots of patient DNA were subjected to polymerase chain reaction (PCR) amplification. These PCR products were each directly DNA sequenced by BigDye Terminator v3.1 cycle sequencing kit (catalog number 4336917; Applied Biosystems, Foster City, CA, USA) with the primers used for PCR amplification; the test showed a positive BRCA1 mutation. According to guidance on genetic testing, we offered genetic counseling services for adult members of her family who wished to know if they had inherited the gene mutation, but none of them chose to undergo any genetic testing.
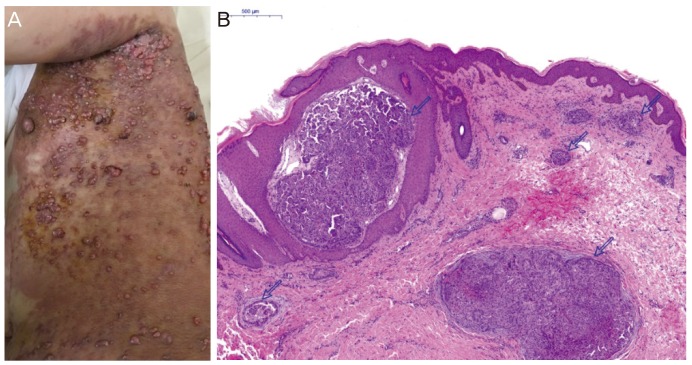
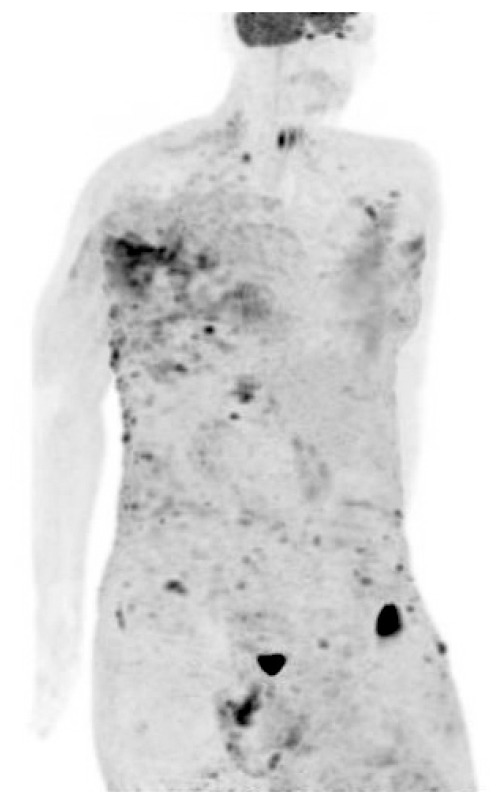
The patient had undergone optimal cytoreductive surgery 5 years ago, followed by adjuvant chemotherapy with paclitaxel and carboplatin. In March 2013, after a 12-month disease-free interval, follow-up positron emission tomography-computed tomography (PET-CT) showed significant progression of peritoneal carcinomatosis. Therefore, a secondary cytoreductive procedure was performed. The patient's chemotherapy regimen had been changed twice based on the results of drug resistance assays. While receiving her latest treatment (cisplatin, doxorubicin, and cyclophosphamide every 3 weeks), blisters appeared on the skin in the upper aspect of the left flank and upper part of the left arm. Multiple large areas with an erythematous vesicular appearance that resembled herpes zoster lesions were noted. In the same areas, multiple new ulcerated, erythematous, nodular skin lesions (1 to 2 cm in diameter) were also observed (Fig. 1A). Biopsy of the skin lesions revealed metastasis of a papillary-type adenocarcinoma in the dermis (Fig. 1B). Cancer antigen (CA)-125 level performed at that time was 269.7 U/mL. Unfortunately, a few days later, a PET scan revealed extensive cutaneous metastasis involving the whole body (Fig. 2), with metastatic lymph nodes on both sides of the neck and in the supraclavicular, axillary, peri-aortic, and right external iliac areas. Based on this information, docetaxel and carboplatin were chosen as the fifth-line chemotherapy agents. However, no significant response was observed after 2 cycles, and in May 2016, 5 years after the diagnosis of OC, and 2 months after diagnosis of the skin metastasis, the patient died.
Approximately 8%–13% of patients diagnosed with epithelial OC have a germ-line BRCA1 or BRCA2 mutation [8]. BRCA1- and BRCA2-mutated OCs have been known to be part of characteristic ‘BRCA syndrome,’ with clinicopathological features such as younger age at onset, advanced stage at diagnosis, a high-grade serous histologic classification, high probability of durable remission after platinum chemotherapy, and a better overall prognosis [91011]. Yang et al. [12] reported that OCs with BRCA2 mutations have better survival rates and better responses to platinum chemotherapy than do OCs with BRCA1 mutations or serous OCs with wild-type BRCA genes.
Herein, we present a case of skin metastases from an ovarian seromucinous adenocarcinoma with a BRCA1 mutation. Cutaneous metastases from internal malignant neoplasms are relatively uncommon. Moreover, skin metastases in BRCA-mutated OCs have not yet been reported. According to a recent study, hereditary BRCA-mutated OCs metastasize to the viscera more frequently than do BRCA-wild-type OCs, implying that direct contiguous spread to the viscera is a feature of the BRCA syndrome phenotype [13]. However, our findings of the presence of multiple skin lesions in the upper regions of the left flank and arm, but not the abdomen, do not exclude the possibility of a tumor embolus spreading through a lymphatic channel or hematogenous routes.
Skin lesions in patients with OC may be an early manifestation of an internal malignant neoplasm and an indicator of metastasis after treatment of the primary cancer [14]. Although it is not easy to think of these skin lesions as a metastasis, a biopsy of the suspected skin lesion in a patient with OC should be considered when its cause is not clear. Moreover, because this maculopapular lesion with multiple vesicles on an erythematous rash closely resembles a herpes zoster infection, a skin biopsy is especially necessary unless there is a definitive diagnosis of a herpetic viral infection. Of course, in most cases, it is difficult to determine the primary origin of cutaneous metastases based only on histopathological findings. Therefore, ultimately, patient characteristics and clinical features should be considered jointly to diagnose the origin of the disease.
Cutaneous metastases, in almost all carcinomas, usually appear late in the progression of the disease and usually have a poor prognosis [7]. There is no standard treatment for OCs with diffuse skin metastases. When the metastases from OC are extensive, electrocoagulation or external radiotherapy may be useful for local control of pain, hemorrhage, and infection. However, treatment of the primary cancer in the pelvic cavity is the overriding consideration. Poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors have cytotoxic effects in BRCA-deficient cells [15]. Although the results of current trials it will be available in the near future, they do not sufficiently explain why PARP inhibitors are efficacious in these cells, and further research regarding this issue is required.
In conclusion, in the last 2 decades, significant advances have been made towards elucidating the roles of BRCA1 and BRCA2 and their mutations in the risk and prognosis of epithelial OC. However, there has been no detailed study on the relapse sites or metastatic spread in patients with BRCA-mutated OCs. Herein, we first describe a case of OC with a BRCA1 mutation and skin metastases. Although there have been numerous studies on skin metastasis from OC, genetic tests may not have been performed. Therefore, this finding has potentially important implications for interest in the metastatic patterns of BRCA-mutated OCs and their clinical management.
References
1. Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003; 72:1117–1130. PMID: 12677558.

2. Boyd J, Sonoda Y, Federici MG, Bogomolniy F, Rhei E, Maresco DL, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA. 2000; 283:2260–2265. PMID: 10807385.
3. Rubin SC, Benjamin I, Behbakht K, Takahashi H, Morgan MA, LiVolsi VA, et al. Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N Engl J Med. 1996; 335:1413–1416. PMID: 8875917.
4. Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003; 97:2187–2195. PMID: 12712470.
5. Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of BRCA1 in the DNA damage response to double-strand breaks. Science. 1999; 286:1162–1166. PMID: 10550055.

6. Dauplat J, Hacker NF, Nieberg RK, Berek JS, Rose TP, Sagae S. Distant metastases in epithelial ovarian carcinoma. Cancer. 1987; 60:1561–1566. PMID: 3621129.

7. Spencer PS, Helm TN. Skin metastases in cancer patients. Cutis. 1987; 39:119–121. PMID: 3829718.
8. Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005; 104:2807–2816. PMID: 16284991.
9. Soegaard M, Kjaer SK, Cox M, Wozniak E, Høgdall E, Høgdall C, et al. BRCA1 and BRCA2 mutation prevalence and clinical characteristics of a population-based series of ovarian cancer cases from Denmark. Clin Cancer Res. 2008; 14:3761–3767. PMID: 18559594.

10. Gilks CB, Prat J. Ovarian carcinoma pathology and genetics: recent advances. Hum Pathol. 2009; 40:1213–1223. PMID: 19552940.

11. Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008; 26:5530–5536. PMID: 18955455.
12. Yang D, Khan S, Sun Y, Hess K, Shmulevich I, Sood AK, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011; 306:1557–1565. PMID: 21990299.

13. Gourley C, Michie CO, Roxburgh P, Yap TA, Harden S, Paul J, et al. Increased incidence of visceral metastases in Scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotype. J Clin Oncol. 2010; 28:2505–2511. PMID: 20406939.

14. Kim YJ, Kim SK, Kim HD, Cho MK, Park YL, Lee JS, et al. A case of skin metastasis of ovarian cancer (Mucinous Cystadenocarcinoma). Korean J Dermatol. 2010; 48:342–345.
15. Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005; 434:913–917. PMID: 15829966.

Fig. 1
(A) Multiple ulcerated, erythematous, nodular skin lesions 1 to 2 cm in diameter were also observed over the upper aspect of the left flank and the upper part of the left upper extremity. (B) The dermis of the skin shows multiple metastatic carcinoma nests, showing papillary configuration (open arrow) (H&E, ×40). H&E, hematoxylin and eosin.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download