Abstract
Objectives
Recurrent caries was partly ascribed to lack of antibacterial properties in composite resin. Silver and zinc nanoparticles are considered to be broad-spectrum antibacterial agents. The aim of the present study was to evaluate the antibacterial properties of composite resins containing 1% silver and zinc-oxide nanoparticles on Streptococcus mutans and Lactobacillus.
Materials and Methods
Ninety discoid tablets containing 0%, 1% nano-silver and 1% nano zinc-oxide particles were prepared from flowable composite resin (n = 30). The antibacterial properties of composite resin discs were evaluated by direct contact test. Diluted solutions of Streptococcus mutans (PTCC 1683) and Lactobacillus (PTCC 1643) were prepared. 0.01 mL of each bacterial species was separately placed on the discs. The discs were transferred to liquid culture media and were incubated at 37℃ for 8 hr. 0.01 mL of each solution was cultured on blood agar and the colonies were counted. Data was analyzed with Kruskall-Wallis and Mann-Whitney U tests.
Results
Composites containing nano zinc-oxide particles or silver nanoparticles exhibited higher antibacterial activity against Streptococcus mutans and Lactobacillus compared to the control group (p < 0.05). The effect of zinc-oxide on Streptococcus mutans was significantly higher than that of silver (p < 0.05). There were no significant differences in the antibacterial activity against Lactobacillus between composites containing silver nanoparticles and those containing zinc-oxide nanoparticles.
Dental caries is one of the most common infectious diseases, in which demineralization of hard tooth tissues occurs by the acid produced as a result of fermentation of carbohydrates by bacteria.1 At present, the majority of carious teeth are restored with tooth-colored restorative materials and composite resins due to esthetic reasons. In fact, restorations with composite resins have predominantly replaced dental amalgam restorations.1,2 An important consideration of composite resin restorations is polymerization shrinkage, which produces gap between the restoration and tooth structure, leading to recurrent caries and the final failure of restoration.3 It has been demonstrated that more microbial plaque is formed on the surface of composite resin restorations compared to amalgam and glass-ionomers.4 As a result of the absence of antibacterial activity in composite resins, attempts have been made to incorporate antibacterial agents such as chlorhexidine, methacryloyloxydodecylpyridinium bromide (MDPB) monomer, ursolic acid and chitosan into composite resins and bonding agents to provide therapeutic effects and antibacterial activity.1,5,6,7 One of the methods suggested is addition of certain antibacterial agents at nano levels to dental composite resins.8,9,10
Nanotechnology is a molecular-level technology and is a promising scientific and applied field in today's medicine. Zinc oxide and silver have proper antibacterial activity and when they are converted into nanoparticles their surface-to-volume ratio increases, improving their antibacterial activity.11,12,13 Studies have shown that zinc oxide can inhibit the production of acid by the dental plaque by inhibiting Lactobacillus and Streptococcus mutans.14 In addition, its antibacterial effect on gram-positive and gram-negative bacteria has been demonstrated. As a result, it is used as an antibacterial agent along with citrate in oral hygiene products such as toothpastes and anti-gingivitis agents.15 Metallic silver is another element commonly used in dentistry. Silver has a superior antibacterial activity compared to other metals; it has a strong cytotoxic effect on a broad range of microorganisms in both metallic and ionic forms. This antibacterial activity has a large number of medical and hygienic applications.9,10,16 Several studies have evaluated the cytotoxicity of silver nanoparticles on fungi, protozoa, a number of viruses, and gram-negative and gram-positive bacteria such as Streptococcus mutans, Lactobacillus, E. coil and Staphylococcus aureus, confirming the antibacterial and bactericidal properties of silver nanoparticles.17,18,19 Since silver is effective against Streptococci in the oral cavity and periodontal pathogens and prevents adhesion of bacteria to surfaces and formation of biofilms, it can be used as a useful antibacterial additive to dental materials.9,20 Some studies have reported the antibacterial activity of silver nanoparticles in tooth-colored restorations against oral Streptococci.8,9
If these composite resins are used as liners beneath restorations or cements used to bond orthodontic brackets, the antibacterial effects of composite resins containing metallic nanoparticles might decrease the development of recurrent caries, increase the longevity of tooth restorations, and be effective in decreasing the formation of bacterial biofilms on teeth and restorations. The aim of the present study was to incorporate zinc oxide and silver nanoparticles into the dental composite resins and to evaluate the effect of these particles on the prevention of bacterial aggregation.
The present experiment was carried out on 90 composite resin discs. Flowable composite resin (Opallis, FGM, Joinville, SC, Brazil) was used as control. ZnO nanoparticle powder, with an average particle size of 50 nm (Maleke-Ashtar Faculty of Nano Tech., Isfahan, Iran) and Ag nanoparticles, with an average particle size of 20 nm (Top Nano Tech Co., Taipei, Taiwan), were separately added into the structure of the composite resin in the laboratory in special containers at a concentration of 1% wt. Then a high-speed mixer (DAC FVZ-K 2006, Hauschild Engineering, Hamm, Germany) was used for mixing and homogeneity of the mix. Round molds were prepared from Polyvinyl chloride with a diameter of 4 mm and a thickness of 1 mm. The molds were placed on a glass slab. A spatula was used to place the composite resins into the molds; then another glass slab was placed on the molds. Both sides of the molds were cured for 60 seconds using a light-curing unit (Optilux 501, Kerr, Orange, CA, USA) and incubated in distilled water at 24℃ for 24 hours. Ninety composite resin discs were prepared with 30 discs containing nano-ZnO, 30 discs containing nano-Ag, and 30 discs without nanoparticles. The composite resin surfaces were polished with 600, 800, and 1,200 grit SiC papers (Matador 991A, Soflex, Starcke's Co., Melle, Germany) to remove the resin-laden surface layer.
Scanning electron microscopy with an energy dispersive X-ray analytical system (SEM-EDX) was performed on two samples in each group to confirm the homogeneity of distribution of nanoparticles in the composite resins. Therefore, two composite resin discs were selected in each group and divided into half using a chisel-like blade and the broken surfaces were gold sputter coated (Sputter coater K45OX, EMITECH Ltd., Ashford, England) in a thin 12 - 15 nm layer to prevent the sample surfaces from burning during SEM observation. The broken surfaces of each sample were observed with a scanning electron microscope (TESCAN, VEGA II XMU, Brno - Kohoutovice, Czech Republic) at ×350 (Figure 1). The elemental gold was eliminated from the diagram by the system software.
The following method was used to compare the antibacterial properties of composite resin discs in a direct contact test. Streptococcus mutans bacterial suspension (Persian type culture collection, PTCC 1683) and Lactobacillus bacterial suspension (PTCC 1643) in brain-heart infusion (BHI) broth with concentration of 0.5 McFarland were prepared (1 mL of this solution contains approximately 1.5 × 108 bacteria). Based on a pilot study, since a visual method was used to count bacteria, in order to decrease the number of bacterial colonies and make it easy to count them, 0.5 McFarland suspension was diluted 1,000 times to achieve a concentration of 1.5 × 105 bacteria in 1 mL. The composite resin discs were sterilized in an autoclave. A sampler was used to place 0.01 mL of the bacterial suspension on the surface of the disc samples. Then the samples containing bacterial suspension were incubated in an incubator containing 5 - 10% of CO2 gas for 1 hour at 37℃ to vaporize the water. The samples were placed in test tubes containing sterile 0.5 mL BHI broth and incubated in an incubator containing 5-10% of CO2 gas at 37℃. After 12 hours of incubation, a sterile sampler was used to retrieve 0.01 mL from each liquid culture media to uniformly spread on a blood agar plate (Merke, Damstadt, Germany). The blood agar plates were incubated for 48 hours at 37℃ and then the numbers of bacterial colonies (Colony Forming Unit, CFU) were visually counted.
Kolmogorov-Smirnov test revealed a lack of normal distribution of data. Therefore, non-parametric Kruskal-Wallis and Mann-Whitney U tests were used for data analysis with SPSS 10 statistical software at a 0.05 Significance level.
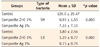
The mean value and standard deviation of the numbers of Streptococcus mutans and Lactobacillus active colonies on surface of blood agar plates in the control group were 126 ± 29.47 and 3.8 ± 2.54, respectively. The mean values and standard deviations of the numbers of Streptococcus mutans and Lactobacillus colonies in the 1 wt% nano zinc oxide group were 7.33 ± 7.19 and 0.73 ± 0.79, respectively, while these means were 0.93 ± 1.53 and 1.2 ± 0.77, in the 1 wt% nano silver group, respectively (Table 1). According to the results of Kruskal-Wallis test, there were significant differences between the control and test groups in relation to the effect on Streptococcus mutans and Lactobacillus (p < 0.05, Table 1). According to the results of Mann-Whitney U test, composite resins containing 1% of zinc oxide nanoparticles and those containing 1% of silver nanoparticles exhibited a significant antibacterial activity against Streptococcus mutans and Lactobacillus compared to the control group (p < 0.05). The effect of incorporating 1% of zinc oxide nanoparticles into composite resin on Streptococcus mutans was significantly higher than that of incorporating 1% of silver nanoparticles (p < 0.05). However, no significant difference was observed between the antibacterial activities of silver and zinc oxide nanoparticles on Lactobacillus.
Nanotechnology is making progress in several scientific fields, including dentistry. In this technology, materials are converted into nanometric sizes in order to produce new properties of the materials.21,22 Antibacterial properties of zinc oxide and silver nanoparticles and their compounds have been used in various medical branches.21,22,23,24 Due to the potential of composite resins to attract microorganisms compared to other restorative materials,9,25 the present study made an attempt to evaluate the antibacterial properties of composite resins containing zinc oxide and silver nanoparticles against Streptococcus mutans and Lactobacillus, which have a significant role in recurrent caries. Silver is a safe bactericidal metal because it is non-toxic to animal cells but is very toxic to bacteria, killing them.23 Although metals and metallic oxides, such as zinc oxide, are considered toxic to human cells at high concentrations, they do not seem to be toxic at very low concentrations.13,26,27
In some studies, diffusion agar disc technique has been used to evaluate antibacterial properties of cured composite resin.24,28,29 However, since composite resins do not secrete any antibacterial agents and the growth inhibitory halo does not form, direct contact technique and BHI broth culture media were used in the present study to evaluate the antibacterial properties of composite resin discs.11,28,29 In some previous studies, spectrophotometry of the liquid of the culture media has been used in order to determine bacterial counts.26 The mechanism of these methods is based on the turbidity of the culture media for the evaluation of antibacterial properties of the materials containing antibacterial particles.26,30 Since active and vital bacteria have a great role in dental caries, without considering vitality of the bacterial cells, determination of bacterial count based on spectrophotometry or direct visualization of bacteria on the surface of composite resin disk under a fluorescent microscope may not be a suitable method. Therefore, in the present study tracing of vital bacterial counts were carried out by placing 0.01 mL of the bacterial solution from each tube on the blood agar culture media since liquid culture media might contain viable and non-viable bacteria.
The results of the present study showed that the number of colonies of viable bacteria in the composite resin plates in the control group was significantly higher than that in the two other groups, demonstrating the bactericidal activity of composite resins containing zinc oxide nanoparticles and silver nanoparticles against Streptococcus mutans and Lactobacillus. Most previous studies have shown the antibacterial activities of zinc oxide and silver nanoparticles incorporated into the composition of composite resins, but there are differing reports about concentration and efficacy of zinc oxide and silver.11,31,32 In the present study, there was a significant difference in the bactericidal activity of composite resins containing 1% of zinc oxide nanoparticles and 1% of silver against Streptococcus mutans, revealing a higher antibacterial activity of zinc oxide against Streptococcus mutans. Contrary to the results of the present study, Hernández-Sierra et al. evaluated the effects of silver (25 nm), zinc oxide (125 nm) and gold (80 nm) nanoparticles on Streptococcus mutans and reported that the antibacterial activity of silver nanoparticles is much higher than those of zinc oxide and gold nanoparticles.8 These differences might be attributed to the size of applied nanoparticles.
Yoshida et al. demonstrated the antibacterial activity of composite resins containing high concentrations of silver nanoparticles (up to 10%) against Streptococcus mutans.31 Spencer used 1.23% and 13% concentrations of zinc oxide nanoparticles for bonding orthodontic brackets to evaluate their antibacterial activity and concluded that zinc oxide can decrease decalcification as a result of orthodontic treatment.32 In the present study, 1% concentration was used in order to avoid the toxicity of silver and zinc and achieve a higher degree of esthetic results in case of silver use. Furthermore, pervious studied showed that incorporation of zinc-oxide or silver nanoparticles up to 1% into the resin composites could significantly inhibit the cariogenic bacteria, without sacrificing the mechanical properties such as flexural strength, flexural modulus, compressive strength and micro-shear bond strength of the resin composites.11,31,33
The addition of water-soluble fluoride releasing filler is another attempt to provide composite resins with antibacterial activity. Fluoride is known to inhibit the biosynthetic metabolism of bacteria. Some studies reported that fluoride releasing resin composites may play an important role in decreasing the cariogenic composition of dental biofilms.34 Van Dijken et al. showed that the fluoride concentrations released from restorations are not enough to affect the metabolism of mutans streptococci in dental plaque.35 Incorporation of inorganic fluoride has resulted in increased fluoride release, but leads to voids in the matrix as the fluoride leaches out of the material.36
Preparation and use of composite resins with antibacterial activity can have a role in improving the clinical results of orthodontic and restorative treatments with composite resins. Orthodontic treatment with fixed appliances makes it difficult for the patient to follow oral hygiene instructions. As a result, caries risk increases due to accumulation of plaque around orthodontic brackets and bonds.37 Recurrent caries and microleakage in posterior composite resin restorations, especially in Class II restorations at cervical areas, are considered important factors in the failure of tooth-colored resin restorations.4 Use of composite resins containing zinc oxide and silver nanoparticles might be effective in reducing the risk of recurrent caries.5,37 In order to preserve the esthetic appearance of restorations it is possible to use bactericidal composite resins as a liner beneath final restorations so that changes in color as a result of incorporation of nanoparticles to composite resin would be minimized. Discoloration due to silver is a major problem with all the silver-containing materials and it especially holds true for tooth-colored composite resins.13 Previous studies have shown that incorporating silver compounds at concentrations higher than 10% into dental materials significantly decreases compressive strength, tensile strength and elastic modulus.9 Use of low concentrations of metallic nanoparticles can prevent severe staining of composite resins.13
Figures and Tables
Acknowledgement
The authors would like to thank the Dental Research Center and Vice Chancellor of Research, Hamadan University of Medical Sciences, for supporting this study.
References
1. Opdam NJ, Bronkhorst EM, Roeters JM, Loomans BA. A retrospective clinical study on longevity of posterior composite and amalgam restorations. Dent Mater. 2007; 23:2–8.

2. Sadowsky SJ. An overview of treatment considerations for esthetic restorations: a review of the literature. J Prosthet Dent. 2006; 96:433–442.

3. Pereira-Cenci T, Cenci MS, Fedorowicz Z, Marchesan MA. Antibacterial agents in composite restorations for the prevention of dental caries. Cochrane Database Syst Rev. 2009; (3):CD007819.

4. Papagiannoulis L, Kakaboura A, Eliades G. In vivo vs in vitro anticariogenic behavior of glass-ionomer and resin composite restorative materials. Dent Mater. 2002; 18:561–569.

5. Thomé T, Mayer MP, Imazato S, Geraldo-Martins VR, Marques MM. In vitro analysis of inhibitory effects of the antibacterial monomer MDPB-containing restorations on the progression of secondary root caries. J Dent. 2009; 37:705–711.

6. Kim S, Song M, Roh BD, Park SH, Park JW. Inhibition of Streptococcus mutans biofilm formation on composite resins containing ursolic acid. Restor Dent Endod. 2013; 38:65–72.

7. Kim JS, Shin DH. Inhibitory effect on Streptococcus mutans and mechanical properties of the chitosan containing composite resin. Restor Dent Endod. 2013; 38:36–42.

8. Hernández-Sierra JF, Ruiz F, Pena DC, Martínez-Gutiérrez F, Martínez AE, Guillén Ade J, Tapia-Pérez H, Castañón GM. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine. 2008; 4:237–240.

9. Bürgers R, Eidt A, Frankenberger R, Rosentritt M, Schweikl H, Handel G, Hahnel S. The anti-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Arch Oral Biol. 2009; 54:595–601.

10. Melo MA, Cheng L, Zhang K, Weir MD, Rodrigues LK, Xu HH. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent Mater. 2013; 29:199–210.

11. Tavassoli Hojati S, Alaghemand H, Hamze F, Ahmadian Babaki F, Rajab-Nia R, Rezvani MB, Kaviani M, Atai M. Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent Mater. 2013; 29:495–505.

12. Xu T, Xie CS. Tetrapod-like nano-particle ZnO/acrylic resin composite and its multi-function property. Prog Org Coat. 2003; 46:297–301.

13. Li LH, Deng JC, Deng HR, Liu ZL, Li XL. Preparation, characterization and antimicrobial activities of chitosan/Ag/ZnO blend films. Chem Eng J. 2010; 160:378–382.

14. Sheng J, Nguyen PT, Marquis RE. Multi-target antimicrobial actions of zinc against oral anaerobes. Arch Oral Biol. 2005; 50:747–757.

15. Cummins D. Zinc citrate/Triclosan: a new anti-plaque system for the control of plaque and the prevention of gingivitis: short-term clinical and mode of action studies. J Clin Periodontol. 1991; 18:455–461.

16. Pinto RJ, Marques PA, Neto CP, Trindade T, Daina S, Sadocco P. Antibacterial activity of nanocomposites of silver and bacterial or vegetable cellulosic fibers. Acta Biomater. 2009; 5:2279–2289.

17. Martinez-Gutierrez F, Olive PL, Banuelos A, Orrantia E, Nino N, Sanchez EM, Ruiz F, Bach H, Av-Gay Y. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomedicine. 2010; 6:681–688.

18. Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004; 275:177–182.

19. Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007; 3:95–101.

20. Spacciapoli P, Buxton D, Rothstein D, Friden P. Antimicrobial activity of silver nitrate against periodontal pathogens. J Periodontal Res. 2001; 36:108–113.

21. Sahoo SK, Parveen S, Panda JJ. The present and future of nanotechnology in human health care. Nanomedicine. 2007; 3:20–31.

22. Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009; 27:76–83.

23. Li LH, Deng JC, Deng HR, Liu ZL, Xin L. Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr Res. 2010; 345:994–998.

24. Kumar R, Münstedt H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials. 2005; 26:2081–2088.

25. Svanberg M, Mjör IA, Orstavik D. Mutans streptococci in plaque from margins of amalgam, composite, and glass-ionomer restorations. J Dent Res. 1990; 69:861–864.

26. Bundy KJ, Butler MF, Hochman RF. An investigation of the bacteriostatic properties of pure metals. J Biomed Mater Res. 1980; 14:653–663.

27. Applerot G, Perkas N, Amirian G, Girshevitz O, Gedanken A. Coating of glass with ZnO via ultrasonic irradiation and a study of its antibacterial properties. Appl Surf Sci. 2009; 256:S3–S8.

28. Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003; 19:449–457.

29. Aydin Sevinç B, Hanley L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J Biomed Mater Res B Appl Biomater. 2010; 94:22–31.

30. Beyth N, Houri-Haddad Y, Baraness-Hadar L, Yudovin-Farber I, Domb AJ, Weiss EI. Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials. 2008; 29:4157–4163.

31. Yoshida K, Tanagawa M, Matsumoto S, Yamada T, Atsuta M. Antibacterial activity of resin composites with silver-containing materials. Eur J Oral Sci. 1999; 107:290–296.

32. Spencer CG, Campbell PM, Buschang PH, Cai J, Honeyman AL. Antimicrobial effects of zinc oxide in an orthodontic bonding agent. Angle Orthod. 2009; 79:317–322.

33. Tanagawa M, Yoshida K, Matsumoto S, Yamada T, Atsuta M. Inhibitory effect of antibacterial resin composite against Streptococcus mutans. Caries Res. 1999; 33:366–371.

34. Pandit S, Kim GR, Lee MH, Jeon JG. Evaluation of Streptococcus mutans biofilms formed on fluoride releasing and non fluoride releasing resin composites. J Dent. 2011; 39:780–787.

35. van Dijken JW, Kalfas S, Litra V, Oliveby A. Fluoride and mutans streptococci levels in plaque on aged restorations of resin-modified glass ionomer cement, compomer and resin composite. Caries Res. 1997; 31:379–383.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download