I. INTRODUCTION
The viability of periodontal ligament (PDL) cells of avulsed or surgically removed teeth has been shown to play a crucial role in success of later replantation or transplantation. Accidentally avulsed or intentionally extracted teeth should be replanted or transplanted as quickly as possible1,2). However, because immediate positioning of teeth is not always possible under different clinical conditions, the preservation of PDL cell viability is a critical problem for successful results of delayed replantation or transplantation. Extended drying time under extra-alveolar condition prior to replantation or transplantation affects PDL cells viability. If teeth with desiccated PDL are replanted or transplanted, common sequelae including root resorption or ankylosis can be followed, which will eventually lead to decreased teeth survival1). Blomlof3) demonstrated that viability of PDL cells remaining on the root surface of avulsed teeth had significantly influenced on replacement resorption after replantation. Thus, the choice of suitable storage medium for maintenance of maximum PDL cells survival until replantation or transplantation is very important.
A number of investigations have performed to find the optimal storage media. Several storage media such as milk, Hank's balanced salt solution, ViaSpan, conditioned medium, and culture medium (Eagle's Medium) were recommended4). Also, some cornea preservation medium such as Optisol-GS or Likorol and chicken egg white were suggested for teeth storage5). However, the results were not consistent.
At present, milk is most recommended as a storage medium of choice for avulsed teeth because of its low cost and the wide availability. Because milk has relatively similar physiological osmolality, optimal pH (approximately pH6.7) and low bacterial contents, it was shown to be effective as storage medium in both clinical and laboratory studies6). It also contains essential nutrients to keep cells viable7). From a practical standpoint, long shelf-life milk was recommended rather than regular pasteurized milk because it is not required to refrigerate at least 6 months8). Harkacz et al.9) also showed that fat content of milk affected PDL cell viability. They suggested that milk with lower fat might be more favorable to preserve cell viability than milk with higher fat.
Another effective storage medium is Hank's balanced salt solution (HBSS). The American Association of Endodontists (AAE) recommends it as the suitable storage medium for preserving avulsed teeth10). It has the optimal pH (approximately pH7) for tissue, and the physiological osmolality of 270 to 290 milliosmoles (mOsm)/L11).
ViaSpan, formulated by Dr. Belzer and his staff in 1979, has been widely used in medicine for flushing and storing of intra-abdominal organs such as kidney, liver and pancreas before transplantation12). Its osmolality is 320 mOsm/L and the pH is approximately 7.47). Previous studies demonstrated that ViaSpan, often referred to as UW (University of Wisconsin) solution, was an effective storage medium for preserving avulsed teeth over a long period of time in spite of its expensiveness, short shelf-life and lack of availability when needed7,13).
Conditioned medium (CM) was introduced for teeth storage14). It is the supernatant of the cultured human PDL fibroblasts. In a study of PDL cell vitality of transplanted dog's teeth, Hupp et al.14) showed that better histologic healing was observed for teeth stored in CM than in HBSS.
On the other hand, Ashkenazi et al.15) showed that culture medium was very effective. They extracted human teeth, scraped off the PDL cells, and cultured them. Plates with confluent PDL cells were soaked in HBSS, culture medium, α-minimal essential medium, and ViaSpan for 2, 8, 24 hours at room temperature. They found that culture medium was the most effective medium to preserve the viability, mitogenicity and clonogenic capacity of PDL cell up to 24 hours at room temperature when compared with HBSS and ViaSpan.
Cell injury may lead to irreversible damages to the cell membrane. It is generally believed that the last pathways of cell injury undergo free radical reactions due to ischemia. In case of the organ transplantation, dysfunction of posttransplant graft also occurs as the result of same mechanism. Organ dysfunction following lung transplantation is mainly caused by ischemia-reperfusion injury16). Free radicals initiate lipid peroxidation of the cell membrane. As the blood flow is reperfused after replantation or transplantation, more free radicals are generated by phagocytes, which lead to cell death including necrosis or apoptosis16,17,18). Cell death is an important factor of transplantation or replantation-related injury16). Optimal storage medium would help to prevent generation of lipid peroxy radicals and protect oxidative damages to the cell membrane18). Thus, the administration of antioxidant for inhibition of generation of these free radicals would decrease the incidence of cell damages following organ transplantation procedures.
Recently, the unknown effect of polyphenol on long-term preservation of rat pancreatic islet cells has been reported18). Polyphenol is known as outstanding antioxidant that is abundant in natural green tea. This has various bioactivities including anticancer function. Hyon et al revealed that islet cells treated with polyphenol could be preserved for over 2 months without any change of the original function and shapes18). The results of this research suggested the possible control of the preservation period by changing the concentration of polyphenol.
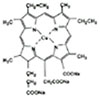
Another potent antioxidant is Chlorophyllin (CHL), which is a mixture of the sodium-copper salts (Fig. 1) and a water-soluble derivatives of green plants pigment chlorophyll. Chlorophyll is a phytochemical which is rich in green vegetables and fruits. Both chlorophyll and CHL are constituents of human diets19,20,21). CHL has been marketed as a deodorant and an accelerant in wound healing as well. It is also used as a food additive for coloration. It has also chemopreventive effects on the growth inhibition of tumor cells20,21,22). CHL was reported to be a highly effective antioxidant, which scavenges various physiologically important reactive oxygen species (ROS)23). These cytoprotective properties of CHL protect cells against oxidative damage. Previous studies have focused mostly on cancer prevention activity of CHL, and not yet demonstrated the potentiality of preservation of PDL cells of avulsed teeth in dentistry. There have been no investigations of the effect of CHL on preservation of the human PDL cell viability associated with replantation or transplantation of teeth.
It was hypothesized that CHL would possess potential effects on preserving PDL cells by antioxidizing against the free radical reactions, on the assumption that functional deterioration of PDL cells of avulsed teeth occurs as a result of the free radical-mediated injury during storage.
The purpose of this in vitro study was first, to evaluate whether CHL could serve as an effective constituent of storage medium to enhance PDL cell viability and second, was to compare the viability of PDL cells after storage in various media.
II. MATERIALS AND METHODS
1. Primary culture of human PDL cells
PDL cells were obtained from fully erupted healthy premolars that were extracted for orthodontic purposes. Scaling and root planing was performed prior to extraction. Teeth were extracted as atraumatically as possible and washed 2 times by phosphate buffered saline (PBS) to eliminate the residual blood. PDL tissues only attached to the middle third of root surface were scraped with #15 scalpel under the aseptic techniques, and then was transferred to 60-mm culture dishes containing 500 µl of F-medium. The F-medium was prepared in our laboratory by mixing of Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Life Technologies, Grand Island, NY, USA) and Ham's nutrient mixtures F-12 (F-12; Gibco-BRL, Life Technologies, Grand Island, NY, USA) at 3:1 ratio. It was also supplemented with a 10% fetal bovine serum and antibiotics consisting of penicillin (100 units/ml), streptomycin (100 µl/ml), and fungizone (0.3 µg/ml) (Gibco-BRL, Life Technologies, Grand Island, NY, USA). After the cells attached to the dishes, 5 ml of F-medium was added. The cells were incubated at 37℃ in humidified atmosphere of 5% CO2 and 95% air for 2~4 weeks until a confluent monolayer was formed. The medium was refed every 3 days. Once the sufficient cell proliferation was obtained, culture medium was removed and the cells were rinsed 3 times with PBS. The cells were then trypsinized (1 ml 0.05% trypsin + 0.53 mM EDTA) and transferred into 60-mm culture dish. After the cells reached confluence, they were trypsinized, placed into 100-mm culture dishes and subcultured. PDL cells between the third and seventh passages were used in this experiment in order to achieve their maximum proliferative potential and homogeneity.
2. Preliminary experiment
A preliminary experiment was conducted to determine 50% survival time period of PDL cells in HBSS. HBSS was used as a standard medium on the basis of the guideline of AAE9). HBSS (Gibco-BRL, Life Technologies, Grand Island, NY, USA) was composed of 8 g/L sodium chloride (NaCl), 1 g/L Glucose, 0.4 g/L potassium chloride (KCl), 0.35 g/L sodium bicarbonate (NaHCO3), 0.048 g/L sodium phosphate (Na2HPO4), 0.06 g/L potassium phosphate (KH2PO4), 0.14 g/L calcium chloride (CaCl2), 0.1 g/L magnesium chloride (MgCl2-6H20) and 0.1 g/L magnesium sulfate (MgSO4-7H2O). In order to assess the cell viability, MTT (3,4[4,5-dimethylthiazol-2-y1]-2,5-diphenyltetrazolium bromide) assay was performed. Stored cells were washed three times in PBS and trypsinized. The detached cells were transferred to a test tube containing 5 ml of fresh F-medium, and centrifuged at 4℃, 1500 rpm for 3 minutes. Then, supernatant fluid was removed and collected PDL fibroblasts resuspended in 1 ml of fresh F-medium to obtain single cell suspension. Cells were then counted using hemocytometer. An average of the five readings were used to determine the number of cells per microliter. According to the determined cell concentration, cells were plated into 96-well plates at a concentration of 1×104 cells / 200 µl in each well. The 6 samples were prepared for each column. After incubated at 37℃ in 5% CO2 and 95% air for 24 hours to allow the cells to attach to the plates, the medium was removed and the cells were rinsed 3 times with PBS. 200 µl of HBSS were added to each well and the cells were cultured respectively at 37℃ in 100% humidified air with 5% CO2 for time periods of 0, 6, 8, 10, 12 hours.
At each designated time, HBSS was removed and MTT assay was conducted as follows: 200 µl of the yellow MTT solution (0.05 mg/ml; Sigma Chemical Co., St. Louis, MO, USA) was added to each well. The plates were covered with aluminium-foil, then incubated at 37℃ in 5% CO2 and 95% air for 3 hours. After incubation, purple formazan salt crystals were formed. Untransformed MTT that remained in the supernatant was removed by aspiration. The formed formazan crystals were dissolved by adding 150 µl of dimethylsulfoxide (DMSO; Sigma Chemical Co., St. Louis, MO, USA). The plates were then placed into an ELISA reader (Benchmark Microplate Reader, Bio-Rad, California, USA), and the optical density of the plates was read at a wavelength of 570 nm to measure the maximal absorbance of the solubilized formazan product. For each time period, the mean value of optical density was calculated. An increase in number of living PDL cells in the samples correlates directly to the amount of purple formazan crystals formed. A percentage of the mean optical density at each 6, 8, 10, 12 hours divided by the mean optical density at 0 hour (100% living cells) represents cell viability of HBSS.
3. Main experiment
Based on the results of preliminary experiment, main experiment was performed. This was to compare the efficacy of six different storage media: F-medium (Gibco-BRL, Life Technologies, Grand Island, NY, USA), ViaSpan (DuPont Pharmaceutics, Wilmington, DE, USA), Likorol (Laboratories Chauvin OPSIA, France), and HBSS supplemented with CHL (Sigma Chemical Co., St. Louis, MO, USA) at 3 different concentrations. Powdered CHL was dissolved in HBSS (Gibco-BRL, Life Technologies, Grand Island, NY, USA). HBSS-only served as a positive control. Tested storage media were divided into 6 groups as follows: the group A: F-medium, the group B: ViaSpan, the group C: Likorol, the group D: HBSS supplemented with 10 nM of CHL, the group E: HBSS supplemented with 100 nM of CHL, the group F: HBSS supplemented with 500 nM of CHL.
MTT assay
For the experiments, a 96-well tissue culture plate (Young Science. Seoul, Korea) was plated with 200 µl of test storage medium containing 1×104 cells / 200 µl in each well. The periodontal cells were then incubated in each tested media respectively for 6 hours. After the incubation of all samples at 37℃, MTT assays were repeated 3 times following the assay protocol described in the preliminary experiment. The mean optical density value and the percentage of viable cells were calculated.
Flow cytometry
Flow cytometry was carried out to determine the different phases of the treated cell cycle. Treated cells with HBSS-only, F-medium, HBSS supplemented with 500 nM of CHL were harvested by trypsinization. Cells were washed twice with PBS and resuspended in 500 µl of ice-cold ethanol and kept at 4℃ for at least 30 minutes. Prior to analysis, cells were washed again with ice-cold PBS, resuspended in 500 µl of ice-cold PBS and treated with 0.1 mg/ml RNase A (Sigma Chemical Co., St. Louis, MO, USA) at 37℃ for 30 min. Propidium iodide (PI; Sigma Chemical Co., St. Louis, MO, USA) at a final concentration of 50 µg/ml was then added to the cell suspensions. After incubation on ice for 30 min, cells were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA) equipped with Argon laser at 488 nm. The percentages of cells in G0-G1, S, and G2-M phase were estimated under the assumption of the Gaussian distribution. The multicycle mode FIT 2.0 cell cycle software (version 2.0, Verity Software House Inc., Topsham, ME, USA) was used to calculate the fraction of cells in G0-G1 (represented by the first peak on the histogram), S (between the first peak and second peak), and G2-M (the second peak) phases. The protocol was repeated 3 times per each group. The means and standard deviations were determined.
III. RESULTS
1. Time period of 50% survival of PDL cells in HBSS
In a preliminary experiment, 50% of the cells were viable after 6-hour storage in HBSS, and cell viability was subsequently decreased over time. 15% of the cells remained vital at 24-hour time period (Fig. 2, 3-a). At 72-hour time period, no cells survived (Fig. 3-b). Thus, this 6-hour time period served as a standard of storage time for different media.
2. The viability of PDL cells
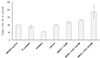
The results of the viability test are shown in Figure 4. The results indicate that HBSS with CHL 500 nM was significantly (p≤0.05) better in maintaining PDL cell viability than the other groups, except for HBSS with CHL 100 nM. Except for HBSS with CHL 500 nM, the other tested media were not significantly different (p>0.05) from the control (HBSS). The number of viable cells in ViaSpan dropped to 56%, but the difference between ViaSpan and the positive control was not statistically significant (p>0.05). The addition of CHL to HBSS showed better cell viability dose-dependently, although there were no statistical differences.
3. Flow cytometry
The results of flow cytometric analysis are summarized in Fig. 5. Cell cycle phase distribution-DNA content histogram and dot-plot profiles by flow cytometric analysis are shown in Fig. 6, 7, 8. The percentage of cells in G0-G1, S, G2-M phases of the cell cycle was estimated in each of 3 different media. PDL cells of all test groups were determined to consist of 77~80% in G0-G1 phases, 11~13% in S phase, and 7~12% in G2-M phases. These results showed that more than 77% of PDL cells consisted in G0-G1 phases of the cell cycle. The apoptotic or necrotic cells were not found.
IV. DISCUSSION
This in vitro study was designed first to evaluate the potential effect of CHL added to HBSS when compared to HBSS without CHL, and second to determine the ability of various media such as F-medium, ViaSpan, and Likorol for maintenance of human PDL cell viability. The viability of cultured PDL cell was evaluated after storage in different media for 6 hours at 37℃. MTT assay and flow cytometric technique were used to assess cellular viability and cell cycle analysis, respectively.
MTT assay used in this study is a reliable method to assess viability of cells. It is more sensitive and precise in reflecting both integrity of cell membrane and cellular metabolic status than a trypan blue exclusion test24). This technique is based on reduction of the yellow tetrazolium salt 3,4[4,5-dimethylthiazol-2-y1]-2,5-diphenyltetrazolium bromide (MTT) to purple formazan crystals by the mitochondrial dehydrogenase enzymes (MTT reductase) in metabolic cells. These formazan crystals are stored in the cytoplasm of living test cells. The optical density of formazan crystals represents maximum peak at a wavelength of 570 nm, which is corresponding to the number of viable cell populations24). Flow cytometry is a useful technique for cell cycle analysis. It has been used to evaluate the quantitative distribution of PDL cells in each of the G0, G1, S, G2, and M phases of the cell cycle. The cell cycle is the sequential process of cell growth, DNA synthesis, and cell division. This method relies on the measurement of DNA content of cell using fluorescent DNA dye such as PI. Because the principle of this analysis is based on the characteristics of quantitative binding of PI to internucleosomal DNA, the cell's position in the cycle can be estimated25). Therefore, DNA content analysis provides estimates of cell percentages in G0-GI, S, and G2-M phase of the cell cycle. The G0 (G zero) phase is a state of cell resting (quiescence). G0 cells may exit the cycle at G1 and enter a stage designated G0. Resting cells maintain their functions. However, G0 cells can reenter G1 of the cell cycle with proper stimulation. The G1 (Gap1) phase is characterized by gene expression and protein synthesis, which primes the cell to enter the next phase: S (Synthesis of DNA) phase. The first peak and the second peak on the DNA content histogram represent G0-G1 and G2-M, respectively25).
The results of the present study suggested that the addition of CHL to HBSS would be beneficial to improve its ability to maintain PDL cell viability. CHL treated cells showed the dose-dependent response to its concentrations. F-medium and Likorol also appeared to be suitable for maintenance of PDL cell viability.
Clinical situations associated with the replantation or transplantation of avulsed teeth were usually restricted by time. Therefore, clinical success or failure depends on whether the extraoral storage of teeth minimizes the deleterious effects until the repositioning into the recipient region. In cases of avulsion accompanied with contusion or fracture of alveolar socket, immediate replantation of teeth would be postponed. When the teeth located in fracture lines of the jaw should be extracted prophylactically, the teeth should be stored properly during the healing period. It might also be necessary to store third molars or first premolars extracted for orthodontic reasons for extended periods of time until later transplantation. A cryopreservation technique has been attempted for these purposes26,27). The PDL cells of cryopreserved human teeth in liquid nitrogen at - 196℃ could survive at least 18 to 54 months in a frozen state26). Schwartz26) documented first human case demonstrating the satisfactory healing on autotransplantation of cryopreserved teeth for 18 months. However, long-term storage of teeth by cryopreservation technique is of limited benefits on practical use due to difficult access including technical problems and a high cost of employment. These methods have not yet gained wide clinical acceptance in storing avulsed or surgically removed teeth intact for extended period of time. From the practical point of view, an important step towards the goal of long-term preservation of teeth would be the development of more effective, simple and available storage medium, which is capable of preserving vital cells for at least days or weeks rather than hours.
A number of studies have examined the usefulness on several storage media. Tap water, saliva, and saline were ineffective on the maintenance of PDL cell vitality. These media have not been recommended any more because of its hypotonic property or a high incidence of bacterial contamination, leading to rapid death of PDL cells28). Krasner and Rankow4) dictated that the best storage media for PDL cells were cell-supporting solutions such as HBSS, ViaSpan, and culture medium. The AAE recommended HBSS as the storage medium of choice for avulsed teeth in 199410). According to this clinical guideline, HBSS was commercially available as emergency tooth preserving system such as: Save-A-Tooth (3M Health Care, St. Paul MN, USA) or Teeth Saver 'Neo' (Neo dental chemical products co., Japan). HBSS has advantages of relative inexpensiveness and long shelf life of 2 years at room temperature29). Hiltz and Trope13) showed that ViaSpan was an effective storage medium. They demonstrated that HBSS was able to maintain vitality of human lip fibroblasts in culture for 24 hours, with 71.3% vital cells remaining. They also revealed that viable cells in HBSS at 48-hour time period dropped to 38.0%, and no cells survived by 120 hours. Huang et al.30) also showed HBSS best compared to milk, contact lens solution, and saline, with 46.8% of vital human PDL cells remaining after 72 hours. On the other hand, in vitro study of Ashkenazi et al.15) revealed that culture medium was more effective than HBSS and ViaSpan. In this study, HBSS was capable of preserving 50% of the PDL cells in culture for up to 6 hours, and dropped to less than 35% at 12 hours. At 24 and 48-hour time periods, 15% and 1% of viable cells remained respectively. At 72-hour, there was no vital cell remaining in HBSS. This result was different from previous report by Hiltz and Trope13). These differences might be attributed to the different origin of the cells and assay methods, or the biopsy site. Hiltz and Trope13) used lip fibroblasts instead of PDL cells, and Huang et al.30) determined viability of cells depending on their morphologic changes and loss of attachment capacity to the culture dishes. Despite morphologic similarities of fibroblasts from different sources, human lip, gingival, and PDL fibroblasts were not functionally identical in culture8,11,25,31). Periodontal connective tissue fibroblasts are not homogenous but have different shapes, sizes, and functional activities25,31). Therefore, Olson et al.11) dictated that a research of storage media for avulsed teeth should not use human lip fibroblast (or dog's PDL cells) but human PDL fibroblasts. It was also shown that in vitro assay of cell attachment efficiency was not sensitive to evaluate the effectiveness of storage media7,32,33).
The present experiment showed that ViaSpan was inferior to the other media. This result was not in agreement with previous studies13,14). Hiltz and Trope13), in a study using replanted dogs' teeth soaked in three media after extended extraoral dry times, demonstrated that ViaSpan was superior to milk, and similar to HBSS to maintain PDL cell viability. Pettiette et al.29) also showed that ViaSpan was more beneficial to an increased healing incidence for teeth that have been dry for 45 or 60 minutes than HBSS. Therefore, they recommended clinically that teeth drying for 45-60 min should be replanted after soaking in ViaSpan for 30 min. On the contrary, Ashkenazi et al.15) demonstrated that culture medium was the most effective up to 24 hours compared to HBSS, ViaSpan. Although the cell viability in ViaSpan and F-medium (modified formulation of culture medium) was lower than HBSS in this investigation, this difference was not of statistical significance. It seemed that the lower ability of Fmedium might be due to relatively short storage time period. In addition, it was presumed that the decreased cell viability in ViaSpan might be attributed to the higher storage temperature than room temperature or 4℃. ViaSpan is originally intended for hypothermic storage of organ. Thus, this solution is recommended to use after precooling to about 2-6℃ in ice, and the organ storage container should be maintained surrounding by ice. However, in our experiment, all the viability tests were performed at 37℃ to provide the optimal culturing condition. This higher storage temperature might have influenced the efficacy of ViaSpan. Glutathione (GSH) is one of the components of ViaSpan, which functions as antioxidant. Although this agent was relatively impermeable across the cell membrane, it could possibly get into the cell and prevent cell injury in the cold34). Therefore, it seemed that the storage at 37℃ might inhibit the activity of GSH, and lead to the decreased viability of PDL cells in ViaSpan.
Likorol is a corneal preservation medium such as Optisol-GS (Chiron-Intra Optics, Inc., Irvine, California, USA) and Likorol-DX (Laboratories Chauvin OPSIA, France). They contain different concentrations of chondroitin sulfate35). Halberstadt et al.35) reported that storage for up to 6 days in Likorol did not significantly decrease in cell density of pig's corneal endothelium. Our study using human PDL cells showed that Likorol might be also useful as teeth storage medium.
The addition of CHL to HBSS showed better cell viability dose-dependently (Fig. 5). Significant differences were found in HBSS supplemented with 500 nM of CHL when compared with the control and other lower CHL concentration groups. We do not have the concrete answer how the CHL helped the cell viability but presume that this element might have reduced the potentials of free radical damage to the cultured PDL cells.
Free radical-mediated cell injury is a mechanism proposed to cause injury to the preserved organs either during preservation or after transplantation16,17,18). Free radicals play a significant pathological role in numerous human diseases. These molecules, so called ROS, have been implicated in control of cellular metabolism. Either losing or gaining an electron resulting in unpaired electrons generates free radicals. Examples of free radicals are oxygen-centered radicals (superoxide, hydroxyl), sulphur-centered radicals (thiyl), carbon-centered radicals (trichloromethyl) and nitric oxide. Recently, an attention has concentrated on oxygen-centered radicals. They are very reactive and capable of propagating further oxidation by chain reactions with other less reactive types. The resulting chain reaction compounds increase the potential of cellular damage. Highly reactive radicals especially such as hydroxyl attack biological molecules including lipid by abstraction of hydrogen, which is a mechanism of triggering lipid peroxidation of cell membrane. Lipid peroxidation is initiated by the attack of free radicals on a polyunsaturated fatty acid becoming oxidized lipid peroxides. Lipid peroxides are toxic and able to lead to cell membrane failure36,37,38). Suppressing the generation of oxygen free radicals may improve an effectiveness of organ preservation to prevent lipid peroxidation. Compounds scavenging free radicals are called antioxidants, which inhibit the generation of them. In an investigation of the effect of tea on the serum lipid peroxidation, Tewari et al.39) showed that polyphenol as antioxidant nutrients provided the protection against free radical reactions. Hyon et al.18) also found that polyphenol treatment markedly enhanced survival rates of pancreatic islets cell, which may be effective on control of long-term preservation of islets cell. Kamat et al.40) showed that CHL had an effective antioxidant ability to inhibit lipid peroxidation, even at a low concentration of 10 micromoles. It was also shown that there was a significant reduction in potentials of oxidative damage in cell suspensions from liver, brain and testis of mice, which fed on CHL at a dose of 1% in drinking water.
The mean viability in HBSS supplemented with 500 nM of CHL showed a relatively large standard deviation. As concentration of CHL is increased, green pigments of CHL might have influence upon assessment of MTT technique. Despite of serial dilution, it was presumed that green color of CHL might interfere with colorimetric detection, which would result in the decreased cell viability.
In this study using flow cytometric method, there were no cells manifested cell death including apoptosis or necrosis, and 80% of PDL cells stored in HBSS supplemented with 500 nM of CHL were in G0-G1 phase. This result suggested that most cells were in a stationary or stable state. Therefore, supplementation of 500 nM of CHL to HBSS would provide more favorable environments to PDL cells than HBSS alone.
In conclusion, the addition of CHL to HBSS may be of help in maintaining the PDL cell viability. Within the scope of this experiment, the effect of CHL was dose-dependent. However, further research is needed to investigate the potential effect of CHL treatment in vivo associated with storage temperature, and to quantify its optimal concentration for the preservation of the PDL cell viability of avulsed teeth.




 PDF
PDF ePub
ePub Citation
Citation Print
Print









 XML Download
XML Download