Abstract
As dental implant surgery and bone grafts were widely operated in Korean dentist, many bone substitutes are commercially available, currently. For commercially used in Korea, all bone substitutes are firstly evaluated by the Ministry of Health and Welfare (MOHW) for safety and efficacy of the product. After being priced, classified, and registration by the Health Insurance Review and Assessment Service (HIRA), the post-application management is obligatory for the manufacturer (or representative importer) to receive a certificate of Good Manufacturing Practice by Ministry of Food and Drug Safety. Currently, bone substitutes are broadly classified into C group (bone union and fracture fixation), T group (human tissue), L group (general and dental material) and non-insurance material group in MOHW notification No. 2018-248. Among them, bone substitutes classified as dental materials (L7) are divided as xenograft and alloplastic bone graft. The purpose of this paper is to analyze alloplastic bone substitutes of 37 products in MOHW notification No. 2018-248 and to evaluate the reference level based on the ISI Web of Knowledge, PubMed, EMBASE (1980–2019), Cochrane Database, and Google Scholar using the criteria of registered or trademarked product name.
As dental implant surgery for edentulous patients became a gold standard, bone grafts such as guided bone regeneration and sinus lift were widely operated in Korean dentist. There has been increased in the number of bone substitute products available to the dental clinician. Still the autologous bone is considered to gold standard, because of its three properties with osteoconduction, osteoinduction and osteogenesis. Osteogenesis is, the property of autogenous graft, generation of new bone from osteogenic cells within the graft. Osteoinduction is the property of the autogenous graft, allogenic graft and intrinsic bone matrix proteins such as transforming growth factor and bone morphogenetic proteins (BMP) to recruit of host stem cells. Osteoconduction is the property of a mechanical structure with biocompatibility for the migration of osteogenetic cells12.
Allograft has been widely used and is an attractive alternative as it avoids donor site morbidity. It has the following advantage: (1) donor site is not needed, (2) abundant supply, and (3) little risk of transmission of infectious diseases3. The ideal alloplastic bone substitutes is biologically stable and maintain its volume with allowing cell infiltration and remodeling process4. The alloplastic bone substitutes has various osteoconductive capabilities depending on the manufacturing methods, crystal structure, size of pores, mechanical properties, composition and absorption rate5.
Hydroxyapatite (HA) is the main mineralized of bone tissue and it exerts an osteoconductive ability when grafted in the defect. Synthetic calcium phosphate ceramics (β-tricalcium phosphate [β-TCP] and HA) could be altered to autogenous graft, allogenic graft and xenogenic graft and it was used as block, cement, pastes, powder, granules and putty type with carboxymethyl cellulose or hyaluronic acid6. In Korea, the development of implant dentistry has led to the development of many dental synthetic bone substitute in many domestic companies.
As dental implant surgery for edentulous patients became a gold standard, bone grafts such as guided bone regeneration and sinus lift were widely operated in Korean dentist. All bone substitutes used commercially in Korea are firstly evaluated by the Ministry of Health and Welfare (MOHW) for safety and efficacy of the product. They are commercialized after being priced, classified, and registration by the Health Insurance Review and Assessment Service (HIRA). The post-application management is obligatory for the manufacturer (or representative importer) to receive a certificate of Good Manufacturing Practice (GMP) by Ministry of Food and Drug Safety (MFDS).
According to Korea Food and Drug Safety (KFDS) No. 2016-156 of ‘medical device manufacturing and quality control standards’, after the approval of commercially use, the manufacturer or importer is required to renew the conformity certification every three years or immediately if the information of product changed7. If any information of the product changed, the certificate of conformity should be issued or reissued by the manufacturer or the importer. Therefore, the manufacturer or importer of registered in the MFDS could be important factors in terms of quality control of currently available bone substitutes.
However, it is difficult for clinicians to know whether the certification or the quality of product is properly managed. Therefore, the purpose of this study is to analyze ingredients, manufacturers, importers, current status and reference levels of dental synthetic bone listed in MOHW notification No. 2018-248.
Commercially available dental alloplastic bone substitute which was approved MOHW notification (No. 2018-248)8 is analyzed the details of manufacturer, importer, composition, available form, Food and Drug Administration (FDA, USA) approval.
This review of literature included studies that detailed the use of bone graft substitute in dental situation, animal, in vivo, and in vitro studies. We excluded studies in the orthopedic and neurosurgery field and those not published in English or Korean. The Google Scholar, ISI Web of Knowledge, PubMed, EMBASE (1980–2019) and Cochrane Databases were searched in February 2019 using the criteria of registered or trademarked product name. The authors read the full text of the studies and classified it according to the ‘level of evidence’ presented by Wright et al.9.(Table 1)
Human study level I evidence is a prospective, randomized, or splint-mouth study with definite results that support the use of alloplastic bone substitute in clinical condition. The case report was classified as level IV. Clinicial studies used alloplastic bone substitute as carrier of osteoinductive growth factors or as comparison of membrane efficacy have not been evaluated for osteoconductive capacity, but have been assigned to human study level IV as showing clinical stability. The animal, in vivo, and in vivo study were separately indicated. All authors reviewed each paper and independently assigned evidence levels. If there is a disagreement on the assigned level, discussion and resolution were made. All studied with human study level I, II, III, or IV were included to be citation10.
In December 2018, thirty-seven dental alloplastic substitutes were registered in MOHW and HIRA8. However, there were two products (BIO-C [Cowellmedi, Busan, Korea] and OssPol-Dental [Genewel, Seongnam, Korea]) that were not commercially available and one product of DualPor COLLAGEN D-INJECTION (OssGen, Daegu, Korea) that was discontinued in the market. Of the remaining 34 alloplastic substitutes, 28 products (82.4%) could be obtained information and included in this review. To approve certificate of GMP from MOHW and MFDS, the company should submit the researches for safety and efficacy of its product, as same procedure as U.S. FDA.(Table 2) The researches, however, were not published and the authors could not include in this review. The available information regarding the delivery form, component, indications, morphology (porosity, biomechanical structure, particle size), and property are shown in Tables 3, 4, 5, 6, 7, 8.
Seven products were approved in FDA11121314151617.(Table 2) Although TCP Dental (Kasios SAS, L'Union, France) was not licensed for dental indication in intended use of FDA17. However, the authors included TCP Dental in this category because manufacturer did not distinguish between KASIOS TCP and (KASIOS) TCP Dental.
The details of dental alloplastic bone substitute which was approved by MOHW notification No. 2018-248 were analyzed8.(Table 3) Among them, BIO-C and OssPol-dental were officially discontinued. CollaOss (SK Bioland, Cheonan, Korea) is registered as 60% of HA, 0.3% of bovine-derived collagen (0.3%) and 39.7% of distilled water and as block, syringe and putty types. Currently, only bock and putty type are available in the manufacturer. DualPor COLLAGEN D-PUTTY and DualPor COLLAGEN D-INJECTION is available as DualPor Collagen D-Putty and DualPor Collagen Injection, but no any information could be found.
There are seven products that do not match the manufacturer or importer registered in MOHW: (1) MBCP+ (Biometlante, Vigneux-de-Bretagne, France; sold only as MBCP and MBCP syringe type, not MBCP+), (2) Excelos Inject (Bio-Alpha, Seongnam, Korea; produced by CGbio, Seongnam, Korea), (3) Excelos (TCPGLD) (BioAlpha; produced by CGbio, sold exclusively by Excelos), (4) Boncel-Os (BioAlpha; produced by CGbio), (5) Mega-TCP (manufactured by CGbio; MegaGen, Seoul, Korea, sold as a single product without discrimination between CGM and CGL), (6) Cerasorb and Cerasorb M granule (sold only by Curasan, Kleinostheim, Germany: Cerasorb M, registered as importer) BI Trading currently available is not available), and (7) OSTEON Sinus (GENOSS, Suwon, Korea: sold as syringe type of OSTEON I, II, or III).(Table 3)
CollaOss is listed as a dental synthetic bone in the MOHW and HIRA data. Although it was represented as xenograft in the journal18192021; however, it was included in this review.(Table 3)
There are three products that do not match in the component registered in MOHW: (1) Cerasorb M granules (99% β-TCP not β-TCP combined with HA; Curasan), (2) FRABONE-Inject (Inobone, Cheonan, Korea: hyaluronic acid addition with HA+β-TCP), and (3) TCP Dental (99% β-TCP not 95% β-TCP combined with 5% HA).(Table 3)
As a result, out of the 33 dental bone substitutes that are currently registered in MOHW and HIRA, 28 products could be commercially available when considering the products that are different form registered information as below: Excelos (TCPGLD) and Excelos (TCPGMD, TCPGLD) are sold exclusively by Excelos, Mega-TCP (CGL) and Mega-TCP (CGM, CGL) are sold only by Mega-TCP, Cerasorb and Cerasorb M granules are sold by Cerasorb M, Cerasorb M is 99% β-TCP, FRABONE-Inject is sold by adding hyaluronic acid, CollaOss is sold in putty and block form without syringe type, DualPor COLLAGEN D-INJECTION is not produced, and TCP Dental (99% β-TCP).
The main components of dental alloplastic bone substitute are tricalcium phosphate (Ca3(PO4)2, β-TCP), calcium phosphate (CaP), and hydroxiapatitie (Ca10(PO4)6(OH)2, HA) which is crystalline form of CaP.(Table 4)
HA is an inorganic material which account for 65% of bone matrix and can be classified as dense and porous, sintered ceramic and non-ceramic, and bovine, coralline and synthetic depending on the origin. Typical characteristics are as below. (1) As large as the particle size, it remains for a long time with slow absorption. (2) The higher the porosity, the easier the penetration of new bone and the quicker absorbed. (3) The larger the crystallinity, the longer the absorption period. (4) Rigid and dense block-form products have high compressive strength but are susceptible to fracture. (5) The higher the porosity, the lower the strength5.
Among the dental alloplastic bone substitutes allowed for use in Korea, there were four products that consisted of HA. OssaBase-HA (LASAK, Praha, Czech) has a retrospective study of guided bone regeneration in 2018, but it was obtained human study level IV due to a poor study design22. However, many other animal, in vivo, and in vitro studies for osteoconductivity23242526. No journals were found for Ovis BONE HA (DENTIS, Seoul, Korea). CollaOss consists of 90% porcine-deriven HA and 10% porcine deriven collagen. It was classified as alloplastic graft in MOHW and HIRA, on the other hands, it was introduced as xenograft in many studies18192021. In the manufacturer (SK Bioland), it is commercially available in plug type and putty type. In comparison with the collagenated bovine bone (Bio-Oss collagen; Geistlich Biomaterials, Woulhusen, Switzerland) into the extraction socket, it was received the human study level III because there was no difference in the efficacy18. Human study level IV was received in a clinical study to comprare the effects of membranes on peri-implant defect19. Animal studies showed osteoconductivity2021.(Table 4)
TCP has a composition of calcium and phosphorus in ratio of 3 and 2. It was known as partially transition into HA and absorption in vivo, but various absorption periods of three to 24 months have been reported depending on the products. The rate of absorption varies according to the chemical structure, porosity and particle size of the material5. The general characteristics suggested by the manufacturer of TCP are as follows. (1) Use with platelet-rich plasma is effective. (2) It is absorbed at the same time as new bone graft. (3) Due to the interconnection of the pores, bone fibers are rapidly penetrated and could promote the regeneration. (4) Since the particle is rounded, there is little mechanical irrigation in surrounding tissues and little inflammatory reaction. (5) High mechanical stability prevents early collapse and inhibits undesirable macrophage activity.
Of the approved products for Korean dental alloplastic bone substitute, seven products that consist with TCP were commercialization. BoneSigma TCP (SigmaGraft, Fullerton, CA, USA) has been described as one of the in vitro studies27, and clinically available products2829 but no clinical studies have been published. Excelos is registered as β-TCP etc. in MOHW and has two types of powder and injection and registered. Injection type is a mixture of biodegradable polymers such as poloxamer and hydroxypropyl methylcellulose (HPMC) to enhance injectable property, moldability and hemostasis. A clinical study comparing putty type Excelos with extraction and using as BMP carriers received a human study level IV30. Excelos has animal studies for BMP carrier3132 and in vivo study for osteoconduction33. No journals were found for Mega-TCP. Sorbone (META-BIOMED, Cheongju, Korea) was validated and received human study level II by a split-mouth study as a control of cockle-shell bone substitute in socket preservation34, and used as a control material for the effect of alendronate on periodontal intra-osseous defect35. SynCera (Oscotec, Seongnam, Korea) had animal and in vivo study for osteoconductivity3637. Cerasorb was approved by the FDA and commercially available to Cerasorb M which reduced porosity from 80% to 65%11. It was received human study level I by randomized controlled trial and systematic review that was equivalent to an autogenous graft in sinus lift3839. It was received human study level III in cystic lesion, periodontal defect and cleft alveolus40. Also, as a result of histologically sufficient alveolar bone regeneration, human study level III was obtained in extraction socket41. As human study level IV, it was used with an enamel matrix derivative in the periodontal defect4243, peri-implant defect after immediately implantation after extraction44, every lots of animal, in vivo, and in vitro studies for osteoconduction6454647484950515253545556. TCP Dental was registered as 5% of HA and 95% of β-TCP in MOHW and HIRA. However, the manufacturer (Kasios SAS) and importer (B.IMTECH, Yongin, Korea) advertised as 99% of β-TCP. Many studies and FDA 510(k) also represented as β-TCP17575859606162636465. It was received human study level III by successful histologic and clinical result comparing Xenograft (BonePlus-xs; Integros, Adana, Turkey) in sinus lift57. Animal, in vivo, and in vitro studies for osteoconductivity5859606162636465.
The mixing ratio of HA and TCP varies from 2:8 to 7:3. It has the following characteristics. (1) It has micropore and macropore. They could induce effective tissue reaction and growth of new bone tissue. Micropores could enable ion exchange and form new contact surfaces for cell adhesion through the deposition of bone crystals. Macropores could help in angiogenesis and remodeling and growth of new bone. (2) HA acts as a mechanical support until the new bone tissue could be remolded for structural stability, and TCP could spread the adhesion surface of osteoblast by ion exchange through rapid resorption. (3) It has porosity of 70% to 90%5.
Boncel-Os (CGbio) consists with 30% of HA and 70% of β-TCP. It was introduced as one of the clinically available products66, and there is an animal study used as a BMP carrier56. BoneSigma BCP (SigmaGraft) consists with 60% of HA and 40% of β-TCP. In vivo study has been published that it inhibited osteoclast formation with plate-rich fibrin67. Dualpore Collagen D-Putty was registered as 60% of HA, 0.3% of bovine-derived collagen, and 39.7% of distilled water by the MOHW and HIRA. On the other hand, the manufacturer (OssGen) advertised the product as 60% HA and 40% β-TCP in 94.5% of biphasic CaP, and with an additional 5.5% bovine collagen but there are no reports of any evaluations of the evaluating its information provided. In MOHW and MFDS, Inobone has registrated its products as FRABONE DENTAL and FRABONE DENTLA INJECT, but they are commercially available as FRABONE and FRABONE-Inject. FRABONE (Inobone) consists with 60% of HA and 40% of β-TCP and it was received patent in USA, Germany, and Korea as mimic the harversian canal structure6869. FRABONE-Inject (Inobone) is a product of hyaluronic acid addition to FRABONE, which advertised to increase moldability and absorption rate by act as soluble granules of hyaluronic acid. However, there was no related researched were found. GENESISBCP (DIO, Busan, Korea) consists with 60% of HA and 40% of β-TCP. It was received human study level II by prospective controlled clinical trial which results good outcome in periodontal defect70. In horizontal augmentation, it was showed successful result with NanoBone (HA and silica gel matrix; Artoss GmbH, Warnemünde, Germany) in case report and it obtained human study level IV71. There were many animal, in vivo, and in vitro studies for osteoconductivity727374757677. Manufacturers have the two types of MBCP as combination of HA and β-TCP as ratio as 60:40 and 20:80, and a moldable MBCP (In'Oss) made by mixing hydrogel to MBCP. However, represented importer (Purgo Biologics, Seongnam, Korea) only has granule or syringe type of MBCP+ which consists with 20% of HA and 80% of β-TCP. MBCP and MBCP+ were received in FDA 510(k) approved1216. There were many clinical studies from MBCP which consists with 60% of HA and 40% of β-TCP78798081828384. On the other hand, MBCP+ was published only animal studies85868788 and in vitro studies8990. Although not introduced as MBCP+, a combination of 20% of HA and 80% of β-TCP was as same resorption and bone growth as combination of 60% of HA and 40% of β-TCP in retrospective clinical trial for extraction socket and it could be human study level IV84. Newbone (GENOSS) consists with 20% of HA and 80% of β-TCP. Although there were animal and in vivo studies for osteoinductivity919293, no clinical studies were found. Boncel-Os consists with 30% of HA and 70% of β-TCP. It was introduced as one of clinically available products66 and used as BMP carrier in animal study56.
OSTEON series (GENOSS) were available as vial, sinus, and lift type. OSTEON, OSTEON II, and OSTEON III were received FDA 510(k) approval131415. In registration of MOHW and HIRAO, OSTEON and OSTEON Sinus were separated but OSTEON Sinus is not available product to commercially use. The manufacture (GENOSS) has classified OSTEON Sinus and OSTEON Lifting according to the size of syringe. In FDA 510(k), there also received approval as same as OSTEON, OSTEON Sinus, and OSTEON Lifting13. OSTEON consists with 70% of HA and 30% of β-TCP. In retrospective clinical study for sinus lift, OSTEON alone could result well-developed lamellar bone as same as Xenograft (Bio-Oss; Osteohealth, Shirley, NY, USA) and it could be received human study level III94. There were many animal and in vivo studies for osteoconductivity95969798. OSTEON II consists with 30% of HA and 70% of β-TCP. In retrospective clinical study for extraction socket, OSTEON II and OSTEON II Collagen were significantly more effective than collagen or native defect and the histological result was shown in animal studies99. Therefore, it could be received human study level III in extraction socket. It was received human study level IV by retrospective study as control group for sinus lift100, 6 months after vertical augmentation which particulated OSTEON II was showed no significantly difference on volume change and peri-implant marginal bone loss compared with autogenous block and allogenous block bone101, successful results on clinical and histologically in ridge augmentation102103, successful outcome on graft after implant removal104, and clinically effective on periodontal defect105. Many animal and in vivo studies for osteoconductivity959697106107. OSTEON III consists with 60% of HA and 40% of β-TCP. There was animal study as BMP carrier108. Although there was no OSTEON III Collagen related study, OSTEON II Collagen had animal and in vivo studies for osteoconductivity109110. Ovis BONE BCP (DENTIS) consists with 20% of HA and 80% of β-TCP. No journals were found for Ovis BONE BCP. Q-Oss+ (OSSTEM IMPLANT, Seoul, Korea) consists with 20% of HA and 80% of β-TCP. It was received human study level IV by the clinical study on peri-implant defect111. There were in vivo study for osteoconductivity112 TOPGEN-S (Toplan, Seoul, Korea) consists 20% of HA and 80% of β-TCP and there could not be found for journals for TOPGEN-S.
Commercially available dental alloplastic bone substitute which was approved MOHW notification No. 2018-248 were broadly divided into 4 groups as C group (bone union and fixation group), L group (general materials), T group (human tissue), and non-insurance group. In the subcategory, there were C0 group (bone substitutes: xenograft, alloplastic graft), L7 group (dental material: dental xenograft, dental alloplastic graft), TB group (bone, demineralized bone matrix, bone block, bone chip, bone powder), non-insurance group (treatment material, human-derived bone, bone substitute containing bone morphogenetic protein [rhBMP-2])8. Among them, dental alloplastic bone substitutes in L7 of L group were included in this study.
The post-application management is obligatory for the manufacturer (or representative importer) to receive a certification of GMP by MFDS. According to FKDS No. 2016-156 of ‘medical device manufacturing and quality control standards’, the certification of GMP of human tissue or functional replacement product should be renewed every three years in article 9. According to article 10 of KFDS No. 2016-156, the certification of GMP should be reissued when any information for the products changed (change of the name of the importer or manufacturer, change of location of the importer or manufacturer). In article 12 and 15, the quality control examination agency reports periodic on the compatibility of medical device to director of KFDA7. Therefore, the manufacturer or importer of registered in the MFDS could be important factors in terms of quality control of currently available bone substitutes.
However, nineteen products (51.4%) were different information among the 37 products registered in MOHW. Four products (10.8%) were different registered ingredients from journal or advertisement including DualPor COLLAGEN D-PUTTY (OssGen), Cerasorb M granules, FRABONE-Inject, and TCP Dental. Nine products (24.3%) were differ in product name or not available including CollaOss (Syringe), Mega-TCP (CGL), Cerasorb, Cerasorb M granules, BIO-C, Excelos (TCPGMD, TCPGLD), MBCP Plus (Biometlante), OssPol DENTAL, and OSTEON Sinus. Especially, CollaOss (Block) and CollaOss (Putty) were registered as dental alloplastic bone substitute in MOHW but they were introduced as xenograft in advertisement and journals. Five products (13.5%) had different manufacturer or importer including Excelos Inject (CGbio), Excelos (TCPGLD), Excelos (TCPGMD, TCPGLD), Mega-TCP (CGM, CGL), Boncel-Os.
For a successful clinical outcome, it cannot be overemphasized that the quality of the materials or medical device should be constant and strictly controlled. Unfortunately, it is hard to identify the certification of GMP or to verify the quality in every clinical situation. Therefore, it is necessary to leave certificate to the government agency or the company which is responsible for the product. In addition the related dental institute or academy should to consider the security on quality of the product.
Implant dentistry has become a common treatment in Korea, many studies and development have been made on implant and bone graft materials. Among dental alloplastic bone substitutes which were registered in MOHW, twenty-nine (78.4%) products were domestically produced, of which three out of seven approved by FDA were made in Korea11121314151617. However, there are only ten products (27.0%) have been published with clinical study, of which six are Korean products. In the view of reference, the reference level could not be as directly same as the efficiency of the product, but it could be the basis of product selection for the clinician since minimal safety and efficiency can be regarded as verified. Reference level I received Cerasorb M (β-TCP 99%) as a sinus lift3839. Reference level II received Sorbone (β-TCP 100%) in extraction socket and periodontal defect3435, GENESIS-BCP (β-TCP 40% and HA 60%) in periodontal defect70. Reference level III received Cerasorb M (β-TCP 99%) in cystic cavity, periodontal defect, cleft defect and extraction socket4041, CollaOss (HA 90% and collagen 10%) in extraction socket18, OSTEON (β-TCP 30% and HA 70%) in sinus lift94, OSTEON II (β-TCP 70% and HA 30%) in sinus lift99, TCP Dental (β-TCP 99.9%) in sinus lift57. Reference level IV is insufficient to verify the efficiency, could be seen as a step that clinically confirms safety. Cerasorb M was in peri-implant and periodontal defect424344, CollaOss was in peri-implant defect19, OssaBase-HA (HA 100%) was in guided bone regeneration22, Excelos (β-TCP 100%) was in extraction socket30, MBCP+ (β-TCP 80% and HA 20%) was in extraction socket84, GENESISBCP was in ridge augmentation71, OSTEON II was in sinus lift100, ridge augmentation101102103, periodontal defect105 achieved for reference level IV. In addition, there were many animal, in vivo, and in vitro studies for osteoconductivity or role as carrier of osteoinductive growth factors or control material. In order to obtain MOHW and MFDS approval for commercial use in Korea, a data based on research or experiments should be required, but these data could not be included in this study because they were not publicly available. Because dental bone graft surgery has been performed in various environments such as sinus lift, ridge augmentation, cystic lesion, periodontal defect, peri-implant defect, extraction socket, it could be difficult to obtain high reference level in all dental bone grafting fields. However, it is nevertheless necessary to demonstrate the clinical level of Korean dental operation and the development level of bone graft substitutes.
In conclusion, there is not enough information about the effectiveness and safety of currently available alloplastic bone substitute in dental performance. Further clinical trials including well designed RCTs are necessary to evaluation the clinical efficacy of dental alloplastic bone substitutes in Korea. It should be aware of the limited information and developed the clinical evidences and regulations for clinicians.
Notes
Authors' Contributions: J.K.K. performed study, participated in data collection and wrote the manuscript. I.H. attributed to write the manuscript. B.K.L. and P.Y.Y. analyzed the study, J.K.L. helped in drafting the manuscript and helped in study design. All authors read and approved the final manuscript.
References
1. Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005; 36:1392–1404. PMID: 16102764.

2. Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: what they can offer and what they cannot. J Bone Joint Surg Br. 2007; 89:574–579. PMID: 17540738.
3. Dalkýz M, Ozcan A, Yapar M, Gökay N, Yüncü M. Evaluation of the effects of different biomaterials on bone defects. Implant Dent. 2000; 9:226–235. PMID: 11307409.

4. Kim YK, Yun PY, Lim SC, Kim SG. Sinus bone graft using OSTEON® and BioOss®: histologic comparative study. Implantology. 2007; 11:4–18.
5. Kim YK. Systematic classification and application of alloplastic bony substitutes and autogenous teeth bone graft material. J Dent Implant Res. 2009; 28:77–88.
6. Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials. 2004; 25:987–994. PMID: 14615163.

7. Ministry of Food and Drug Safety. Medical device manufacturing and quality control standards (No. 2016-156) [Internet]. Sejong: National Law Information Center;cited 2017 Mar 1. Available from: http://www.law.go.kr/LSW/admRulLsInfoP.do?admRulSeq=2100000073289.
8. Ministry of Health & Welfare. No. 2018-248. Medical device price list [Internet]. Wonju: Health Insurance Review & Assessment Service;cited 2018 Nov 30. Available from: https://www.hira.or.kr/rd/insuadtcrtr/bbsView.do?pgmid=HIRAA030069000400&brdScnBltNo=4&brdBltNo=51151.
9. Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003; 85:1–3.

10. Kurien T, Pearson RG, Scammell BE. Bone graft substitutes currently available in orthopaedic practice: the evidence for their use. Bone Joint J. 2013; 95:583–597. PMID: 23632666.
11. U.S. Food and Drug Administration (FDA). Cerasorb: 510(k) summary [Internet]. Silver Spring (MD): FDA;cited 2017 Sep 17. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf11/K113282.pdf.
12. U.S. Food and Drug Administration (FDA). MBCP+: 510(k) summary [Internet]. Silver Spring (MD): FDA;cited 2007 Jul 30. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf9/K093122.pdf.
13. U.S. Food and Drug Administration (FDA). Osteon: 510(k) summary [Internet]. Silver Spring (MD): FDA;cited 2010 Jul 8. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf10/K102015.pdf.
14. U.S. Food and Drug Administration (FDA). Osteon II: 510(k) summary [Internet]. Silver Spring (MD): FDA;cited 2012 Jan 17. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf11/K112716.pdf.
15. U.S. Food and Drug Administration (FDA). Osteon III: 510(k) summary [Internet]. Silver Spring (MD): FDA;cited 2016 Sep 14. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf15/K153676.pdf.
16. U.S. Food and Drug Administration (FDA). MBCP: 510(k) summary [Internet]. Silver Spring (MD): FDA;cited 2005 May 3. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf5/K051885.pdf.
17. U.S. Food and Drug Administration (FDA). Kasios TCP: 510(k) summary [Internet]. Silver Spring (MD): FDA;cited 2004 Nov 10. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf4/K042340.pdf.
18. Lim HC, Jung UW, You H, Lee JS. Randomized clinical trial of ridge preservation using porcine bone/cross-linked collagen vs. bovine bone/non-cross-linked collagen: cone beam computed tomographic analysis. Clin Oral Implants Res. 2017; 28:1492–1500. PMID: 28370361.

19. Lee JH, Lee JS, Baek WS, Lim HC, Cha JK, Choi SH, et al. Assessment of dehydrothermally cross-linked collagen membrane for guided bone regeneration around peri-implant dehiscence defects: a randomized single-blinded clinical trial. J Periodontal Implant Sci. 2015; 45:229–237. PMID: 26732806.

20. Benic GI, Joo MJ, Yoon SR, Cha JK, Jung UW. Primary ridge augmentation with collagenated xenogenic block bone substitute in combination with collagen membrane and rhBMP-2: a pilot histological investigation. Clin Oral Implants Res. 2017; 28:1543–1552. PMID: 28574217.

21. Kwak EJ, Cha IH, Nam W, Yook JI, Park YB, Kim HJ. Effects of locally administered rhBMP-2 and bisphosphonate on bone regeneration in the rat fibula. Oral Dis. 2018; 24:1042–1056. PMID: 29582561.

22. Papež J, Dostálová T, Chleborád K, Kříž P, Strnad J. Chronological age as factor influencing the dental implant osseointegration in the jaw bone. Prague Med Rep. 2018; 119:43–51.

23. Petrenko YA. Properties of mesenchymal stromal cells during 3D culturing within scaffolds of different origin. Probl Cryobiol. 2012; 22:144–147.
24. Horkavcová D, Zítková K, Rohanová D, Helebrant A, Cílová Z. The Resorption of ß-TCP and HA materials under conditions similar to those in living organisms. Ceram Silik. 2010; 54:398–404.
25. Strnadová M, Strnad Z, Šponer P, Jirošova J, Strnad J. In vivo behaviour of the synthetic porous hydroxyapatite prepared by low temperature microwave processing and comparison with deproteinized bovine bone. Key Eng Mater. 2012; 493-494:236–241.
26. Rohanová D, Horkavcová D, Helebrant A, Boccaccini AR. Assessment of in vitro testing approaches for bioactive inorganic materials. J Non-Cryst Solid. 2016; 432:53–59.

27. Lee DSH, Pai Y, Chang S. Effect of thermal treatment of the hydroxyapatite powders on the micropore and microstructure of porous biphasic calcium phosphate composite granules. J Biomater Nanobiotechnology. 2013; 4:114–118.

28. Dorozhkin SV. Calcium orthophosphate-based bioceramics and its clinical applications. In : Kaur G, editor. Clinical applications of biomaterials: state-of-the-art progress, trends, and novel approaches. Cham: Springer;2017. p. 123–226.
29. Dorozhkin SV. Multiphasic calcium orthophosphate (CaPO4) bioceramics and their biomedical applications. Ceram Int. 2016; 42:6529–6554.
30. Lee SM. Clinical evaluation of efficacy and safety of NOVOSIS-inject containing bmp-2 for socket preservation after extraction of a single-rooted tooth. Clin Oral Implant Res. 2018; 29:318.
31. Chang AR, Cho TH, Hwang SJ. Receptor activator of nuclear factor kappa-B ligand-induced local osteoporotic canine mandible model for the evaluation of peri-implant bone regeneration. Tissue Eng Part C Methods. 2017; 23:781–794. PMID: 28741427.

32. Song J, Kim J, Woo HM, Yoon B, Park H, Park C, et al. Repair of rabbit radial bone defects using bone morphogenetic protein-2 combined with 3D porous silk fibroin/β-tricalcium phosphate hybrid scaffolds. J Biomater Sci Polym Ed. 2018; 29:716–729. PMID: 29405844.

33. Park HJ, Min KD, Lee MC, Kim SH, Lee OJ, Ju HW, et al. Fabrication of 3D porous SF/β-TCP hybrid scaffolds for bone tissue reconstruction. J Biomed Mater Res A. 2016; 104:1779–1787. PMID: 26999521.

34. Alharissy M, AbouSulaiman A, Manadili A, Dayoub S. Radio graphic alternations in alveolar bone dimensions following socket preservation using two bone substitutes. J Int Dent Med Res. 2018; 11:906–910.
35. Naineni R, Ravi V, Subbaraya DK, Prasanna JS, Panthula VR, Koduganti RR. Effect of alendronate with β - TCP bone substitute in surgical therapy of periodontal intra-osseous defects: a randomized controlled clinical trial. J Clin Diagn Res. 2016; 10:ZC113–ZC117.

36. Miramond T, Borget P, Baroth S, Guy D. Comparative critical study of commercial calcium phosphate bone substitutes in terms of physic-chemical properties. Key Eng Mater. 2014; 587:63–68.

37. Jang CH, Cho YB, Yang HC, Kim JS, Choi CH, Jang SJ, et al. Effect of piperacillin-tazobactam coated β-tricalcium phosphate for mastoid obliteration in otitis media. Int J Pediatr Otorhinolaryngol. 2011; 75:631–634. PMID: 21388691.

38. Zijderveld SA, Zerbo IR, van den, Schulten EA, ten Bruggenkate CM. Maxillary sinus floor augmentation using a beta-tricalcium phosphate (Cerasorb) alone compared to autogenous bone grafts. Int J Oral Maxillofac Implants. 2005; 20:432–440. PMID: 15973955.
39. Esposito M, Grusovin MG, Felice P, Karatzopoulos G, Worthington HV, Coulthard P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants - a Cochrane systematic review. Eur J Oral Implantol. 2009; 2:167–184. PMID: 20467628.
40. Horch HH, Sader R, Pautke C, Neff A, Deppe H, Kolk A. Synthetic, pure-phase beta-tricalcium phosphate ceramic granules (Cerasorb) for bone regeneration in the reconstructive surgery of the jaws. Int J Oral Maxillofac Surg. 2006; 35:708–713. PMID: 16690249.

41. Horowitz RA, Mazor Z, Miller RJ, Krauser J, Prasad HS, Rohrer MD. Clinical evaluation alveolar ridge preservation with a beta-tricalcium phosphate socket graft. Compend Contin Educ Dent. 2009; 30:588–590. 592594 passimquiz 604, 606. PMID: 19998726.
42. Döri F, Arweiler N, Gera I, Sculean A. Clinical evaluation of an enamel matrix protein derivative combined with either a natural bone mineral or beta-tricalcium phosphate. J Periodontol. 2005; 76:2236–2243. PMID: 16332235.
43. Bokan I, Bill JS, Schlagenhauf U. Primary flap closure combined with Emdogain alone or Emdogain and Cerasorb in the treatment of intra-bony defects. J Clin Periodontol. 2006; 33:885–893. PMID: 17092241.
44. Harel N, Moses O, Palti A, Ormianer Z. Long-term results of implants immediately placed into extraction sockets grafted with β-tricalcium phosphate: a retrospective study. J Oral Maxillofac Surg. 2013; 71:e63–e68. PMID: 23351769.

45. Klein M, Goetz H, Pazen S, Al-Nawas B, Wagner W, Duschner H. Pore characteristics of bone substitute materials assessed by microcomputed tomography. Clin Oral Implants Res. 2009; 20:67–74.

46. Neamat A, Gawish A, Gamal-Eldeen AM. beta-Tricalcium phosphate promotes cell proliferation, osteogenesis and bone regeneration in intrabony defects in dogs. Arch Oral Biol. 2009; 54:1083–1090. PMID: 19828137.
47. Kasten P, Beyen I, Niemeyer P, Luginbühl R, Bohner M, Richter W. Porosity and pore size of beta-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: an in vitro and in vivo study. Acta Biomater. 2008; 4:1904–1915. PMID: 18571999.
48. Bernhardt A, Lode A, Peters F, Gelinsky M. Novel ceramic bone replacement material Osbone® in a comparative in vitro study with osteoblasts. Clin Oral Implants Res. 2011; 22:651–657. PMID: 21044164.

49. Bernhardt A, Dittrich R, Lode A, Despang F, Gelinsky M. Nanocrystalline spherical hydroxyapatite granules for bone repair: in vitro evaluation with osteoblast-like cells and osteoclasts. J Mater Sci Mater Med. 2013; 24:1755–1766. PMID: 23625348.

50. Klein MO, Kämmerer PW, Scholz T, Moergel M, Kirchmaier CM, Al-Nawas B. Modulation of platelet activation and initial cytokine release by alloplastic bone substitute materials. Clin Oral Implants Res. 2010; 21:336–345. PMID: 20074241.

51. Bernhardt A, Lode A, Peters F, Gelinsky M. Comparative evaluation of different calcium phosphate-based bone graft granules -an in vitro study with osteoblast-like cells. Clin Oral Implants Res. 2013; 24:441–449. PMID: 22092911.
52. Ghanaati S, Barbeck M, Orth C, Willershausen I, Thimm BW, Hoffmann C, et al. Influence of β-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010; 6:4476–4487. PMID: 20624495.

53. Handschel J, Berr K, Depprich R, Naujoks C, Kübler NR, Meyer U, et al. Compatibility of embryonic stem cells with biomaterials. J Biomater Appl. 2009; 23:549–560. PMID: 18757497.

54. Zheng H, Bai Y, Shih MS, Hoffmann C, Peters F, Waldner C, et al. Effect of a β-TCP collagen composite bone substitute on healing of drilled bone voids in the distal femoral condyle of rabbits. J Biomed Mater Res B Appl Biomater. 2014; 102:376–383. PMID: 24039106.

55. Bizenjima T, Takeuchi T, Seshima F, Saito A. Effect of poly (lactide-co-glycolide) (PLGA)-coated beta-tricalcium phosphate on the healing of rat calvarial bone defects: a comparative study with pure-phase beta-tricalcium phosphate. Clin Oral Implants Res. 2016; 27:1360–1367. PMID: 26748831.

56. Bernhardt A, Lode A, Peters F, Gelinsky M. Optimization of culture conditions for osteogenically-induced mesenchymal stem cells in β-tricalcium phosphate ceramics with large interconnected channels. J Tissue Eng Regen Med. 2011; 5:444–453. PMID: 20848550.

57. Kurkcu M, Benlidayi ME, Cam B, Sertdemir Y. Anorganic bovinederived hydroxyapatite vs β-tricalcium phosphate in sinus augmentation: a comparative histomorphometric study. J Oral Implantol. 2012; 38:519–526. PMID: 23072285.

58. Khojasteh A, Eslaminejad MB, Nazarian H. Mesenchymal stem cells enhance bone regeneration in rat calvarial critical size defects more than platelete-rich plasma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106:356–362. discussion 363. PMID: 18424120.

59. Badwelan M, Alkindi M, Ramalingam S, Nooh N, Al Hezaimi K. The efficacy of recombinant platelet-derived growth factor on beta-tricalcium phosphate to regenerate femoral critical sized segmental defects: longitudinal in vivo micro-CT study in a rat model. J Invest Surg. 2018; DOI: 10.1080/08941939.2018.1519048. [Epub ahead of print].

60. Giuliani A, Manescu A, Larsson E, Tromba G, Luongo G, Piattelli A, et al. In vivo regenerative properties of coralline-derived (biocoral) scaffold grafts in human maxillary defects: demonstrative and comparative study with beta-tricalcium phosphate and biphasic calcium phosphate by synchrotron radiation x-ray microtomography. Clin Implant Dent Relat Res. 2014; 16:736–750. PMID: 23350548.

61. Arbez B, Kün-Darbois JD, Convert T, Guillaume B, Mercier P, Hubert L, et al. Biomaterial granules used for filling bone defects constitute 3D scaffolds: porosity, microarchitecture and molecular composition analyzed by microCT and Raman microspectroscopy. J Biomed Mater Res B Appl Biomater. 2019; 107:415–423. PMID: 29675998.

62. Emanuel N, Rosenfeld Y, Cohen O, Applbaum YH, Segal D, Barenholz Y. A lipid-and-polymer-based novel local drug delivery system--BonyPid™: from physicochemical aspects to therapy of bacterially infected bones. J Control Release. 2012; 160:353–361. PMID: 22507550.

63. Catros S, Zwetyenga N, Bareille R, Brouillaud B, Renard M, Amédée J, et al. Subcutaneous-induced membranes have no osteoinductive effect on macroporous HA-TCP in vivo. J Orthop Res. 2009; 27:155–161. PMID: 18683892.

64. Salim AS, Al Hijazi A. Evaluation of the effect of synthetic biomaterial (calcium phosphate ceramic) on healing of extracted tooth socket. J Baghdad College Dent. 2010; 22:57–61.
65. Kursun-Çakmak ES, Akbulut N, Öztas DD. Comparative evaluation of the radiopacity of bone graft materials used in dentistry. J Contemp Dent. 2017; 7:150–155.

66. You H, Yoon SR, Lim HC, Lee JS, Jung UW, Choi SH. Bone regenerative efficacy of limited-dose escherichia coli-derived rh-BMP-2 with biphasic calcium phosphate carrier in rabbit calvarial defect model. Implant Dent. 2016; 25:16–23. PMID: 26606286.

67. Kumar A, Mahendra J, Samuel S, Govindraj J, Loganathan T, Vashum Y, et al. Platelet-rich fibrin/biphasic calcium phosphate impairs osteoclast differentiation and promotes apoptosis by the intrinsic mitochondrial pathway in chronic periodontitis. J Periodontol. 2019; 90:61–71. PMID: 29958327.

68. Fabrication method of a novel artificial cortical bone using a multi-pass extrusion process. KR101241642B1 [Internet]. Daejeon: Korean Intellectual Property Office;cited 2012 Feb 6. Available from: http://kpat.kipris.or.kr/kpat/1020100072191.pdf?method=fullText&applno=1020100072191&pub_reg=P.
69. (WO2012015226) Fabrication method of a novel artificial cortical bone using a multi-pass extrusion process [Internet]. Geneva: World Intellectual Property Organization;cited 2012 Feb 2. Available from: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012015226.
70. Lee MJ, Kim BO, Yu SJ. Clinical evaluation of a biphasic calcium phosphate grafting material in the treatment of human periodontal intrabony defects. J Periodontal Implant Sci. 2012; 42:127–135. PMID: 22977742.

71. Alagl AS, Madi M. Localized ridge augmentation in the anterior maxilla using titanium mesh, an alloplast, and a nano-bone graft: a case report. J Int Med Res. 2018; 46:2001–2007. PMID: 29529906.

72. Kim BS, Yang SS, You HK, Shin HI, Lee J. Fucoidan-induced osteogenic differentiation promotes angiogenesis by inducing vascular endothelial growth factor secretion and accelerates bone repair. J Tissue Eng Regen Med. 2018; 12:e1311–e1324. PMID: 28714275.

73. Yang DH, Park HN, Bae MS, Lee JB, Heo DN, Lee WJ, et al. Evaluation of GENESIS-BCP™ scaffold composed of hydroxyapatite and β-tricalcium phosphate on bone formation. Macromol Res. 2012; 20:627–633.

74. Kim BS, Lee J. Enhanced bone healing by improved fibrin-clot formation via fibrinogen adsorption on biphasic calcium phosphate granules. Clin Oral Implants Res. 2015; 26:1203–1210. PMID: 24888232.

75. Seok H, Lee SK, Kim SG, Kang TY, Lee MJ, Chae WS. Migration of alloplastic bone graft material in infected conditions: a case study and animal experiment. J Oral Maxillofac Surg. 2014; 72:1093.e1–1093.e11. PMID: 24709514.

76. Kim BS, Yang SS, Lee J. Precoating of biphasic calcium phosphate bone substitute with atelocollagen enhances bone regeneration through stimulation of osteoclast activation and angiogenesis. J Biomed Mater Res A. 2017; 105:1446–1456. PMID: 28177580.

77. Lee SH, Kim SW, Lee JI, Yoon HJ. The effect of platelet-rich fibrin on bone regeneration and angiogenesis in rabbit cranial defects. Tissue Eng Regen Med. 2015; 12:362–370.

78. Kim MS, Lee JH, Jung UW, Kim CS, Choi SH, Cho KS. A cumulative survival rate of implants installed on posterior maxilla augmented using MBCP after 2 years of loading: a retrospective clinical study. J Korean Acad Periodontol. 2008; 38:669–678.

79. Lee JH, Jung UW, Kim CS, Choi SH, Cho KS. Maxillary sinus augmentation using Macroporous Biphasic Calcium Phosphate (MBCP™): three case report with histologic evaluation. J Korean Acad Periodontol. 2006; 36:567–577.

80. Le Guehennec L, Goyenvalle E, Aguado E, Pilet P, Bagot D'Arc M, Bilban M, et al. MBCP biphasic calcium phosphate granules and tissucol fibrin sealant in rabbit femoral defects: the effect of fibrin on bone ingrowth. J Mater Sci Mater Med. 2005; 16:29–35. PMID: 15754141.

81. Lee JH, Jung UW, Kim CS, Choi SH, Cho KS. Histologic and clinical evaluation for maxillary sinus augmentation using macroporous biphasic calcium phosphate in human. Clin Oral Implants Res. 2008; 19:767–771. PMID: 18705808.

82. Wagner W, Wiltfang J, Pistner H, Yildirim M, Ploder B, Chapman M, et al. Bone formation with a biphasic calcium phosphate combined with fibrin sealant in maxillary sinus floor elevation for delayed dental implant. Clin Oral Implants Res. 2012; 23:1112–1117. PMID: 22892064.

83. Kim CS, Kim SC, Claire D, Elodie S, Daculsi G. Eight-year clinical follow-up of sinus grafts with micro-macroporous biphasic calcium phosphate granules. Key Eng Mater. 2014; 587:321–324.
84. Rodríguez C, Jean A, Mitja S, Daculsi G. Five years clinical follow up bone regeneration with CaP bioceramics. Key Eng Mater. 2008; 361-363:1339–1342.

85. Jégoux F, Goyenvalle E, Cognet R, Malard O, Moreau F, Daculsi G, et al. Reconstruction of irradiated bone segmental defects with a biomaterial associating MBCP+(R), microstructured collagen membrane and total bone marrow grafting: an experimental study in rabbits. J Biomed Mater Res A. 2009; 91:1160–1169. PMID: 19148925.
86. Miramond T, Aguado E, Goyenvalle E, Moreau F, Borget P, Daculsi G. Osteopromotion of biphasic calcium phosphate granules in critical size defects after osteonecrosis induced by focal heating insults. IRBM. 2013; 34:337–341.

87. Pereira RC, Benelli R, Canciani B, Scaranari M, Daculsi G, Cancedda R, et al. Beta tricalcium phosphate ceramic triggers fast and robust bone formation by human mesenchymal stem cells. J Tissue Eng Regen Med. 2019; DOI: 10.1002/term.2848. [Epub ahead of print].

88. Miramond T, Corre P, Borget P, Moreau F, Guicheux J, Daculsi G, et al. Osteoinduction of biphasic calcium phosphate scaffolds in a nude mouse model. J Biomater Appl. 2014; 29:595–604. PMID: 24919403.

89. Houshmand B, Tabibzadeh Z, Motamedian SR, Kouhestani F. Effect of metformin on dental pulp stem cells attachment, proliferation and differentiation cultured on biphasic bone substitutes. Arch Oral Biol. 2018; 95:44–50. PMID: 30048855.

90. Miramond T, Borget P, Baroth S, Daculsi G. Comparative critical study of commercial calcium phosphate bone substitutes in terms of physic-chemical properties. Key Eng Mater. 2014; 587:63–68.

91. Kim KI, Park S, Im GI. Osteogenic differentiation and angiogenesis with cocultured adipose-derived stromal cells and bone marrow stromal cells. Biomaterials. 2014; 35:4792–4804. PMID: 24655782.

92. Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioact Mater. 2017; 2:224–247. PMID: 29744432.
93. Habibovic P, Kruyt MC, Juhl MV, Clyens S, Martinetti R, Dolcini L, et al. Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J Orthop Res. 2008; 26:1363–1370. PMID: 18404698.

94. Kim YK, Yun PY, Kim SG, Lim SC. Analysis of the healing process in sinus bone grafting using various grafting materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:204–211. PMID: 18801669.

95. Lim HC, Kim KT, Lee JS, Jung UW, Choi SH. In vivo comparative investigation of three synthetic graft materials with varying compositions processed using different methods. Int J Oral Maxillofac Implants. 2015; 30:1280–1286. PMID: 26574853.

96. Lim HC, Zhang ML, Lee JS, Jung UW, Choi SH. Effect of different hydroxyapatite:β-tricalcium phosphate ratios on the osteoconductivity of biphasic calcium phosphate in the rabbit sinus model. Int J Oral Maxillofac Implants. 2015; 30:65–72. PMID: 25265122.

97. Kim DM, Nevins ML, Lin Z, Fateh A, Kim SW, Schupbach P, et al. The clinical and histologic outcome of dental implant in large ridge defect regenerated with alloplast: a randomized controlled preclinical trial. J Oral Implantol. 2013; 39:148–153. PMID: 23611677.

98. Lim HC, Hong JY, Lee JS, Jung UW, Choi SH. Late-term healing in an augmented sinus with different ratios of biphasic calcium phosphate: a pilot study using a rabbit sinus model. J Periodontal Implant Sci. 2016; 46:57–69. PMID: 26937294.

99. Abdulghani MM, Farha LS. Clinical and experimental study to evaluate the effect of biphasic calcium phosphate collagen composite (cpcc) on healing of bone defects after oral surgical procedures. Al-Kindy College Med. 2017; 13:11–20.
100. Hussein LA, Hassan TAL. The effectiveness of oxidized regenerated cellulose as a graft material in transalveolar osteotome sinus lift procedure. J Craniofac Surg. 2017; 28:1766–1771. PMID: 28891903.

101. Park YH, Choi SH, Cho KS, Lee JS. Dimensional alterations following vertical ridge augmentation using collagen membrane and three types of bone grafting materials: a retrospective observational study. Clin Implant Dent Relat Res. 2017; 19:742–749. PMID: 28556452.

102. Kim DM, Camelo M, Nevins M, Fateh A, Schupbach P, Nevins M. Alveolar ridge reconstruction with a composite alloplastic biomaterial. Int J Periodontics Restorative Dent. 2012; 32:e204–e209. PMID: 23057064.
103. Chee YD, Seon HK. Increase of the width of peri-implant keratinized tissue using apically positioned flap: case report. J Dent Rehabil Appl Sci. 2013; 29:407–417.

104. Lee JB. Selectable implant removal methods due to mechanical and biological failures. Case Rep Dent. 2017; 2017:9640517. PMID: 28758035.

105. Badiea RA. Evaluation of treatment of intra-bony defects with a mixture of β-tricalcium phosphate - hydroxyapatite granules and oily calcium hydroxide suspension. J Baghdad College Dent. 2013; 25:103–109.
106. Seo GY, Thoma DS, Jung UW, Lee JS. Increasing the tissue thickness at implant sites using guided bone regeneration and an additional collagen matrix: histologic observations in beagle dogs. J Biomed Mater Res B Appl Biomater. 2019; 107:741–749. PMID: 30080303.

107. Bucchi C, Borie E, Arias A, Dias FJ, Fuentes R. Radiopacity of alloplastic bone grafts measured with cone beam computed tomography: an analysis in rabbit calvaria. Bosn J Basic Med Sci. 2016; 17:61–66. PMID: 27968706.

108. Chung SM, Jung IK, Yoon BH, Choi BR, Kim DM, Jang JS. Evaluation of different combinations of biphasic calcium phosphate and growth factors for bone formation in calvarial defects in a rabbit model. Int J Periodontics Restorative Dent. 2016; 36(Suppl):s49–s59. PMID: 27031634.

109. Al Mukhtar YH, Abid WK. Effect of Osteon II collagen with hyaluronic acid and collagen membrane on bone healing process in rabbits: a radiograghical study. Int J Enhanc Res Sci Tech Eng. 2016; 5:36–46.
110. Khojasteh A, Motamedian SR, Rad MR, Shahriari MH, Nadjmi N. Polymeric vs hydroxyapatite-based scaffolds on dental pulp stem cell proliferation and differentiation. World J Stem Cells. 2015; 7:1215–1221. PMID: 26640621.
111. Tallarico M, Xhanari E, Cocchi F, Canullo L, Schipani F, Meloni SM. Accuracy of computer-assisted template-based implant placement using a conventional impression and scan model or digital impression: a preliminary report from a randomized controlled trial. J Oral Sci Rehabil. 2017; 3:8–16.
112. Kang KJ, Lee MS, Moon CW, Lee JH, Yang HS, Jang YJ. In vitro and in vivo dentinogenic efficacy of human dental pulp-derived cells induced by demineralized dentin matrix and HA-TCP. Stem Cells Int. 2017; 2017:2416254. PMID: 28761445.

Table 1
Level of evidence for research questions9
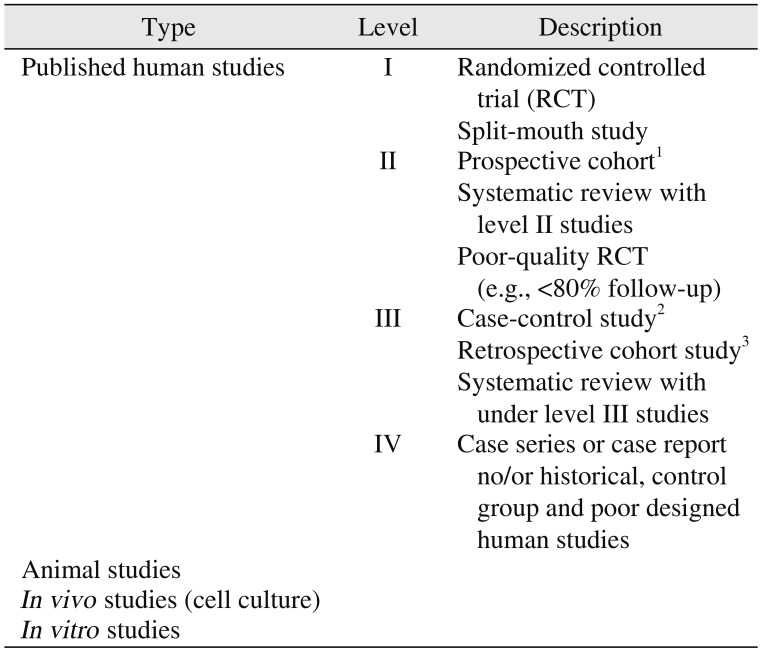
Table 2
Dental bone graft substitutes which Food and Drug Administration (FDA) 510(k) approved
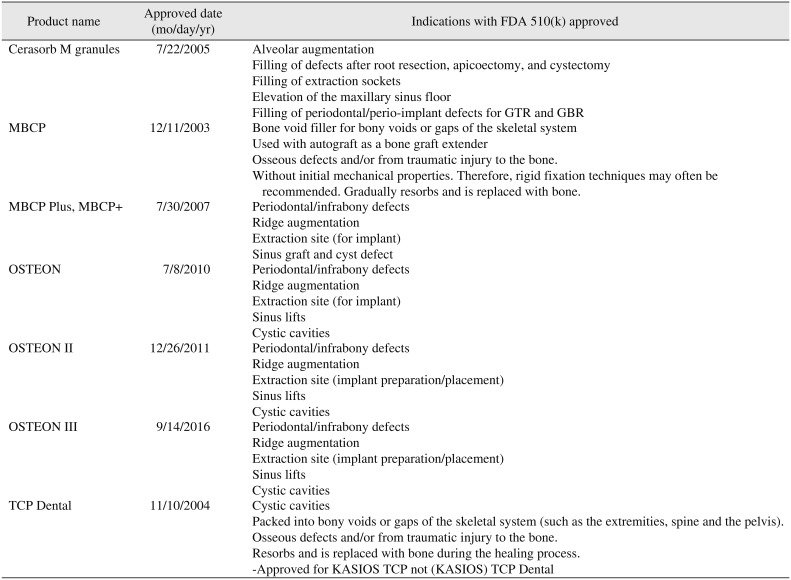
Table 3
Dental bone graft substitutes with manufactures, importer, components and inconsistency with registered in Korean Ministry of Health and Welfare and Korean Health Insurance Review and Assessment Service
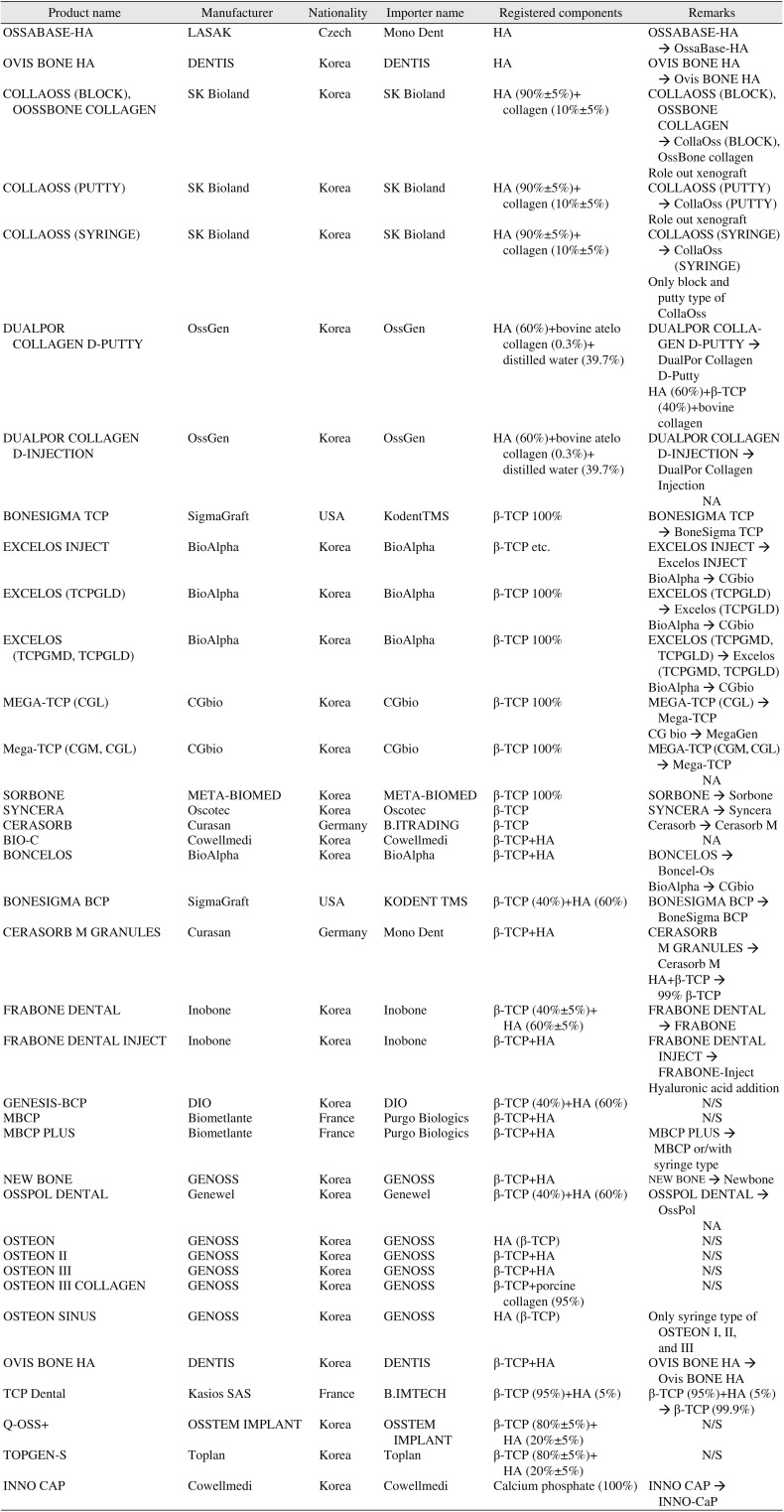
Table 4
Commercially available dental alloplastic bone substitutes according to components
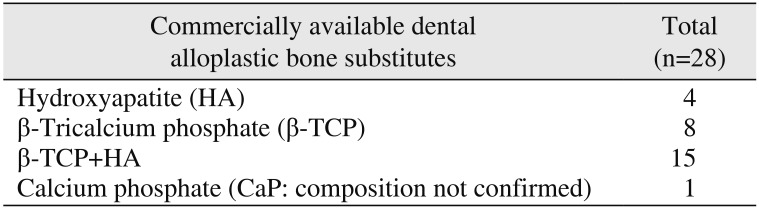
| Commercially available dental alloplastic bone substitutes | Total (n=28) |
|---|---|
| Hydroxyapatite (HA) | 4 |
| β-Tricalcium phosphate (β-TCP) | 8 |
| β-TCP+HA | 15 |
| Calcium phosphate (CaP: composition not confirmed) | 1 |
Table 5
Dental bone graft substitutes which was consisted with hydroxyapatite (HA)
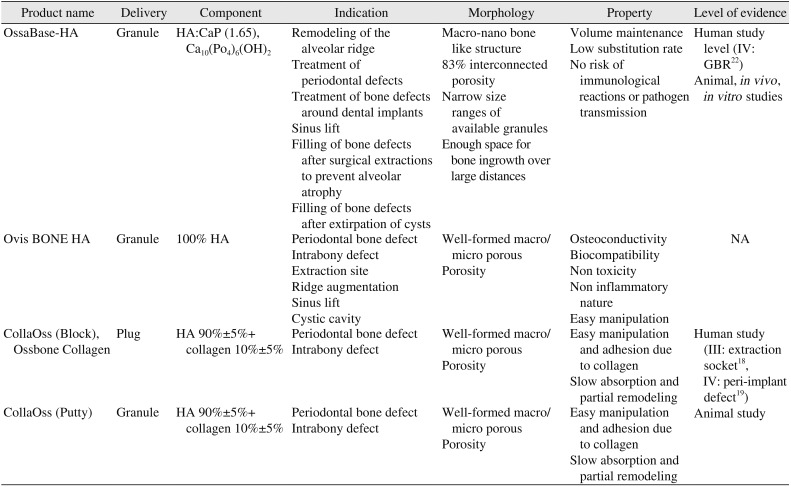
| Product name | Delivery | Component | Indication | Morphology | Property | Level of evidence |
|---|---|---|---|---|---|---|
| OssaBase-HA | Granule | HA:CaP (1.65), Ca10(Po4)6(OH)2 | Remodeling of the alveolar ridge | Macro-nano bone like structure | Volume maintenance | Human study level (IV: GBR22) |
| Treatment of periodontal defects | 83% interconnected porosity | Low substitution rate | Animal, in vivo , in vitro studies | |||
| Treatment of bone defects around dental implants | Narrow size ranges of available granules | No risk of immunological reactions or pathogen transmission | ||||
| Sinus lift | Enough space for bone ingrowth over large distances | |||||
| Filling of bone defects after surgical extractions to prevent alveolar atrophy | ||||||
| Filling of bone defects after extirpation of cysts | ||||||
| Ovis BONE HA | Granule | 100% HA | Periodontal bone defect | Well-formed macro/micro porous | Osteoconductivity | NA |
| Intrabony defect | Porosity | Biocompatibility | ||||
| Extraction site | Non toxicity | |||||
| Ridge augmentation | Non inflammatory nature | |||||
| Sinus lift | Easy manipulation | |||||
| Cystic cavity | ||||||
| CollaOss (Block), Ossbone Collagen | Plug | HA 90%±5%+ collagen 10%±5% | Periodontal bone defect | Well-formed macro/micro porous | Easy manipulation and adhesion due to collagen | Human study (III: extraction socket18, IV: peri-implant defect19) |
| Intrabony defect | Porosity | Slow absorption and partial remodeling | ||||
| CollaOss (Putty) | Granule | HA 90%±5%+collagen 10%±5% | Periodontal bone defect | Well-formed macro/micro porous | Easy manipulation and adhesion due to collagen | Animal study |
| Intrabony defect | Porosity | Slow absorption and partial remodeling |
Table 6
Dental bone graft substitutes which was consisted with β-tricalcium phosphate (β-TCP)
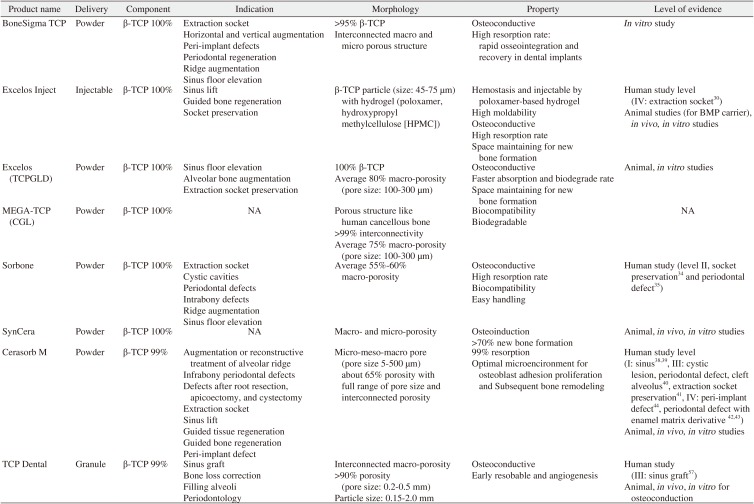
| Product name | Delivery | Component | Indication | Morphology | Property | Level of evidence |
|---|---|---|---|---|---|---|
| BoneSigma TCP | Powder | β-TCP 100% | Extraction socket | >95% β-TCP | Osteoconductive | In vitro study |
| Horizontal and vertical augmentation | Interconnected macro and micro porous structure | High resorption rate: rapid osseointegration and recovery in dental implants | ||||
| Peri-implant defects | ||||||
| Periodontal regeneration | ||||||
| Ridge augmentation | ||||||
| Sinus floor elevation | ||||||
| Excelos Inject | Injectable | β-TCP 100% | Sinus lift | β-TCP particle (size: 45-75 μm) with hydrogel (poloxamer, hydroxypropyl methylcellulose [HPMC]) | Hemostasis and injectable by poloxamer-based hydrogel | Human study level (IV: extraction socket30) |
| Guided bone regeneration | High moldability | Animal studies (for BMP carrier), in vivo, in vitro studies | ||||
| Socket preservation | Osteoconductive | |||||
| High resorption rate | ||||||
| Space maintaining for new bone formation | ||||||
| Excelos (TCPGLD) | Powder | β-TCP 100% | Sinus floor elevation | 100% β-TCP | Osteoconductive | Animal, in vitro studies |
| Alveolar bone augmentation | Average 80% macro-porosity (pore size: 100-300 μm) | Faster absorption and biodegrade rate | ||||
| Extraction socket preservation | Space maintaining for new bone formation | |||||
| MEGA-TCP (CGL) | Powder | β-TCP 100% | NA | Porous structure like human cancellous bone | Biocompatibility | NA |
| >99% interconnectivity | Biodegradable | |||||
| Average 75% macro-porosity (pore size: 100-300 μm) | ||||||
| Sorbone | Powder | β-TCP 100% | Extraction socket | Average 55%-60% macro-porosity | Osteoconductive | Human study (level II, socket preservation34 and periodontal defect35) |
| Cystic cavities | High resorption rate | |||||
| Periodontal defects | Biocompatibility | |||||
| Intrabony defects | Easy handling | |||||
| Ridge augmentation | ||||||
| Sinus floor elevation | ||||||
| SynCera | Powder | β-TCP 100% | NA | Macro- and micro-porosity | Osteoinduction | Animal, in vivo, in vitro studies |
| >70% new bone formation | ||||||
| Cerasorb M | Powder | β-TCP 99% | Augmentation or reconstructive treatment of alveolar ridge | Micro-meso-macro pore (pore size 5-500 μm) about 65% porosity with full range of pore size and interconnected porosity | 99% resorption | Human study level (I: sinus3839, III: cystic lesion, periodontal defect, cleft alveolus40, extraction socket preservation41, IV: peri-implant defect44, periodontal defect with enamel matrix derivative 4243) |
| Infrabony periodontal defects | Optimal microencironment for osteoblast adhesion proliferation and Subsequent bone remodeling | Animal, in vivo, in vitro studies | ||||
| Defects after root resection, apicoectomy, and cystectomy | ||||||
| Extraction socket | ||||||
| Sinus lift | ||||||
| Guided tissue regeneration | ||||||
| Guided bone regeneration | ||||||
| Peri-implant defect | ||||||
| TCP Dental | Granule | β-TCP 99% | Sinus graft | Interconnected macro-porosity | Osteoconductive | Human study (III: sinus graft57) |
| Bone loss correction | >90% porosity (pore size: 0.2-0.5 mm) | Early resobable and angiogenesis | Animal, in vivo , in vitro for osteoconduction | |||
| Filling alveoli | Particle size: 0.15-2.0 mm | |||||
| Periodontology |
Table 7
Dental bone graft substitutes which was composed with hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP)
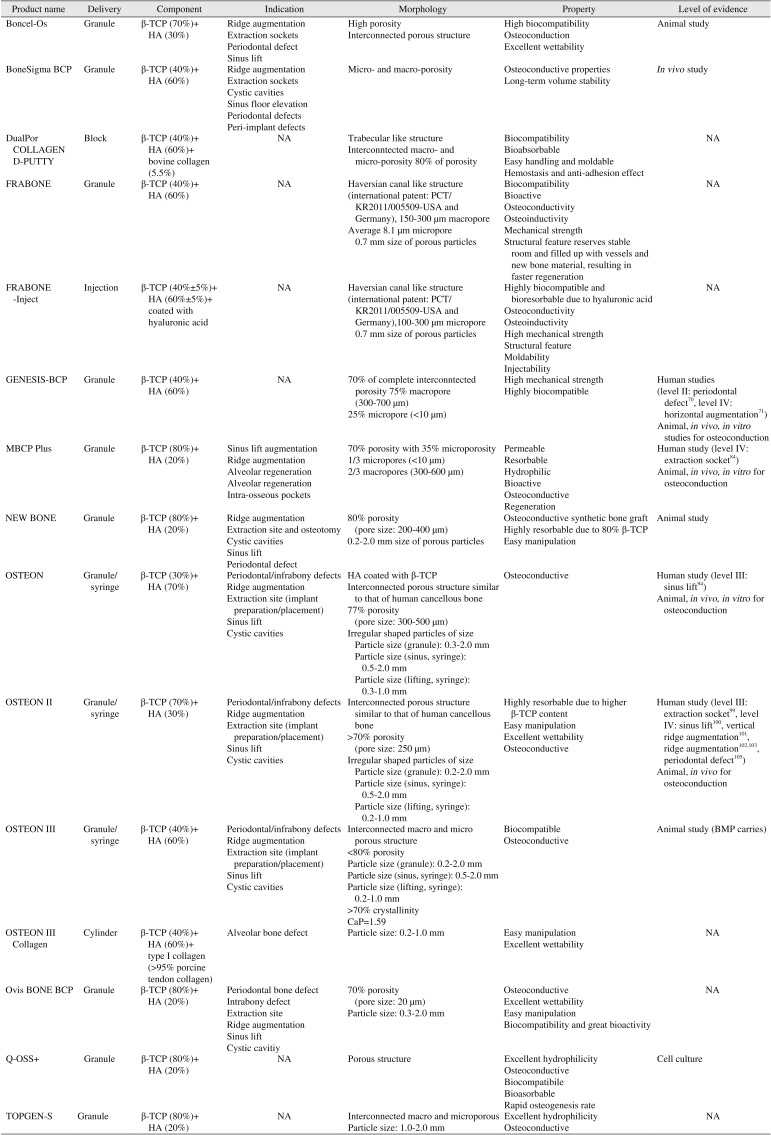
| Product name | Delivery | Component | Indication | Morphology | Property | Level of evidence |
|---|---|---|---|---|---|---|
| Boncel-Os | Granule | β-TCP (70%)+HA (30%) | Ridge augmentation | High porosity | High biocompatibility | Animal study |
| Extraction sockets | Interconnected porous structure | Osteoconduction | ||||
| Periodontal defect | Excellent wettability | |||||
| Sinus lift | ||||||
| BoneSigma BCP | Granule | β-TCP (40%)+HA (60%) | Ridge augmentation | Micro- and macro-porosity | Osteoconductive properties | In vivo study |
| Extraction sockets | Long-term volume stability | |||||
| Cystic cavities | ||||||
| Sinus floor elevation | ||||||
| Periodontal defects | ||||||
| Peri-implant defects | ||||||
| DualPor COLLAGEN D-PUTTY | Block | β-TCP (40%)+HA (60%)+bovine collagen (5.5%) | NA | Trabecular like structure | Biocompatibility | NA |
| Interconntected macro- and micro-porosity 80% of porosity | Bioabsorbable | |||||
| Easy handling and moldable | ||||||
| Hemostasis and anti-adhesion effect | ||||||
| FRABONE | Granule | β-TCP (40%)+HA (60%) | NA | Haversian canal like structure (international patent: PCT/KR2011/005509-USA and Germany), 150-300 μm macropore | Biocompatibility | |
| Average 8.1 μm micropore0.7 mm size of porous particles | Bioactive | |||||
| Osteoconductivity | ||||||
| Osteoinductivity | ||||||
| Mechanical strength | ||||||
| Structural feature reserves stable room and filled up with vessels and new bone material, resulting in faster regeneration | ||||||
| FRABONE-Inject | Injection | β-TCP (40%±5%)+HA (60%±5%)+coated with hyaluronic acid | NA | Haversian canal like structure (international patent: PCT/ KR2011/005509-USA and Germany),100-300 μm micropore 0.7 mm size of porous particles | Highly biocompatible and bioresorbable due to hyaluronic acid | NA |
| Osteoconductivity | ||||||
| Osteoinductivity | ||||||
| High mechanical strength | ||||||
| Structural feature | ||||||
| Moldability | ||||||
| Injectability | ||||||
| GENESIS-BCP | Granule | β-TCP (40%)+HA (60%) | NA | 70% of complete interconntected porosity 75% macropore (300-700 μm) | High mechanical strength | Human studies (level II: periodontal defect70, level IV: horizontal augmentation71) |
| 25% micropore (<10 μm) | Highly biocompatible | Animal, in vivo, in vitro studies for osteoconduction | ||||
| MBCP Plus | Granule | β-TCP (80%)+HA (20%) | Sinus lift augmentation | 70% porosity with 35% microporosity | Permeable | Human study (level IV: extraction socket84) |
| Ridge augmentation | 1/3 micropores (<10 μm) | Resorbable | Animal, in vivo, in vitro for osteoconduction | |||
| Alveolar regeneration | 2/3 macropores (300-600 μm) | Hydrophilic | ||||
| Alveolar regeneration | Bioactive | |||||
| Intra-osseous pockets | Osteoconductive | |||||
| Regeneration | ||||||
| NEW BONE | Granule | β-TCP (80%)+HA (20%) | Ridge augmentation | 80% porosity (pore size: 200-400 μm) | Osteoconductive synthetic bone graft | Animal study |
| Extraction site and osteotomy | 0.2-2.0 mm size of porous particles | Highly resorbable due to 80% β-TCP | ||||
| Cystic cavities | Easy manipulation | |||||
| Sinus lift | ||||||
| Periodontal defect | ||||||
| OSTEON | Granule/syringe | β-TCP (30%)+HA (70%) | Periodontal/infrabony defects | HA coated with β-TCP | Osteoconductive | Human study (level III: sinus lift94) |
| Ridge augmentation | Interconnected porous structure similar to that of human cancellous bone | Animal, in vivo, in vitro for osteoconduction | ||||
| Extraction site (implant preparation/placement) | 77% porosity (pore size: 300-500 | |||||
| Sinus lift | Irregular shaped particles of size | |||||
| Cystic cavities | Particle size (granule): 0.3-2.0 mm | |||||
| Particle size (sinus, syringe): 0.5-2.0 mm | ||||||
| Particle size (lifting, syringe): 0.3-1.0 mm | ||||||
| OSTEON II | Granule/syringe | β-TCP (70%)+HA (30%) | Periodontal/infrabony defects | Interconnected porous structure similar to that of human cancellous bone | Highly resorbable due to higher β-TCP content | Human study (level III: extraction socket99, level IV: sinus lift100, vertical ridge augmentation101, ridge augmentation102103, periodontal defect105) |
| Ridge augmentation | >70% porosity (pore size: 250 μm) | Easy manipulation | Animal, in vivo for osteoconduction | |||
| Extraction site (implant preparation/placement) | Irregular shaped particles of size | Excellent wettability | ||||
| Sinus lift | Particle size (granule): 0.2-2.0 mm | Osteoconductive | ||||
| Cystic cavities | Particle size (sinus, syringe): 0.5-2.0 mm | |||||
| Particle size (lifting, syringe): 0.2-1.0 mm | ||||||
| OSTEON III | Granule/syringe | β-TCP (40%)+HA (60%) | Periodontal/infrabony defects | Interconnected macro and micro porous structure | Biocompatible | Animal study (BMP carries) |
| Ridge augmentation | <80% porosity | Osteoconductive | ||||
| Extraction site (implant preparation/placement) | Particle size (granule): 0.2-2.0 mm | |||||
| Sinus lift | Particle size (sinus, syringe): 0.5-2.0 mm | |||||
| Cystic cavities | Particle size (lifting, syringe): 0.2-1.0 mm | |||||
| >70% crystallinity | ||||||
| CaP=1.59 | ||||||
| OSTEON III Collagen | Cylinder | β-TCP (40%)+HA (60%)+type I collagen (>95% porcine tendon collagen) | Alveolar bone defect | Particle size: 0.2-1.0 mm | Easy manipulation | NA |
| Excellent wettability | ||||||
| Ovis BONE BCP | Granule | β-TCP (80%)+HA (20%) | Periodontal bone defect | 70% porosity (pore size: 20 μm) | Osteoconductive | NA |
| Intrabony defect | Particle size: 0.3-2.0 mm | Excellent wettability | ||||
| Extraction site | Easy manipulation | |||||
| Ridge augmentation | Biocompatibility and great bioactivity | |||||
| Sinus lift | ||||||
| Cystic cavitiy | ||||||
| Q-OSS+ | Granule | β-TCP (80%)+HA (20%) | NA | Porous structure | Excellent hydrophilicity | Cell culture |
| Osteoconductive | ||||||
| Biocompatibile | ||||||
| Bioasorbable | ||||||
| Rapid osteogenesis rate | ||||||
| TOPGEN-S | Granule | β-TCP (80%)+HA (20%) | NA | Interconnected macro and microporous | Excellent hydrophilicity | NA |
| Particle size: 1.0-2.0 mm | Osteoconductive |
Table 8
Dental bone graft substitutes which was consisted with calcium phosphate




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download