Abstract
Hair graying is an obvious sign of human aging. Although graying has been investigated extensively, the mechanism remains unclear. Here, we reviewed previous studies on the mechanism of graying and seek to offer some new insights. The traditional view is that hair graying is caused by exhaustion of the pigmentary potential of the melanocytes of hair bulbs. Melanocyte dysfunction may be attributable to the effects of toxic reactive oxygen species on melanocyte nuclei and mitochondria. A recent study suggests that bulge melanocyte stem cells (MSCs) are the key cells in play. Graying may be caused by defective MSC self-maintenance, not by any deficiency in bulbar melanocytes. Our previous study suggested that graying may be principally attributable to active hair growth. Active hair growth may produce oxidative or genotoxic stress in hair bulge. These internal stress may cause eventually depletion of MSC in the hair follicles. Taken together, hair graying may be caused by MSC depletion by genotoxic stress in the hair bulge. Hair graying may also be sometimes caused by dysfunction of the melanocytes by oxidative stress in the hair bulb. In addition, hair graying may be attributable to MSC depletion by active hair growth.
Hair graying is a common process occurring in people as they age. Fifty percent of the population has about 50% gray hair at the age of 50 years, known as the 50-50-50 rule12. Most non-pigmented hairs are white, attributable to total loss of melanin in the hair bulb. The term ‘gray hair’ refers to an admixture of white non-pigmented hairs and pigmented hairs. Sometimes a single hair fiber can show a progressive dilution of pigment from black, through gray to white. Hair graying may be inherited in an autosomal-dominant manner34. Hair graying usually develops initially at the temples, and then spreads to the frontal area, the vertex, and the parietal region, affecting the occipital region last5. If graying occurs prior to the third decade of life, this is generally termed premature graying, although the criteria may differ56. Hair graying is associated with various autoimmune disorders including vitiligo and several rare premature aging syndromes including the Hutchinson Gilford and Werner'syndrome7. Hair graying may also reflect nutritional deficiencies (especially of vitamins) and the use of drugs such as chloroquine8910. We do not know exactly the cause of hair graying in humans. Some evidences were found in mouse, but still only partial. Here, we reviewed previous studies on the mechanism of graying in humans and seek to offer some new insights.
Tobin and Paus5 proposed that graying is caused by the depletion of hair follicle bulbar melanocytes, due to dysregulation of antioxidant mechanisms and expression of anti-apoptotic factors. Overproduction of copper-zinc superoxidase induces excessive H2O2 formation and triggers oxidative damage. Accumulated reactive oxygen species (ROS) imposes significant oxidative stresses on both bulbar melanocytes per se and the highly proliferative hair bulb epithelium (consisting of keratinocytes)11. Inflammation, ultraviolet light, cigarette smoking, psychoemotional stress, certain chemicals, and genetic defects may trigger oxidative stress, inducing graying121314. It was suggested that graying may be caused by reduced tyrosinase activity in bulbar melanocytes or an abnormal interaction, melanosomes transfer, between these melanocytes and the cortical keratinocytes of hair bulbs1215. Also, graying may result from insufficient melanocyte migration from the reservoir (the hair bulge) of the upper outer root sheath (ORS) to the hair bulb lying closest to the dermal papilla5. The levels of both anti-apoptotic factors (including BCL-2) and melanogenic enzymes (TRP-1 and TRP-2) are valuable markers of graying. The mitochondrion is the primary target of oxidative stressors, compromising energy production. Thus, a mitochondrial theory of aging was suggested to explain hair graying13.
Melanin transfer from melanocytes to cortical keratinocytes may reduce keratinocyte proliferation and increase terminal keratinocyte differentiation, probably by modulating intracellular calcium levels1617. The growth rate of non-pigmented (white) beard hair is greater than that of adjacent pigmented hair18. Melanin granules transferred to keratinocytes may serve as regulatory packages, controlling cell differentiation and metabolic status1920. Together, the data suggest that non-pigmented (white) hairs may grow more rapidly and become thicker than pigmented hairs, due to an absence of melanin in bulbar keratinocytes. Active hair growth of white hairs may be considered as a result, not cause, of hair graying.
Insufficient neuroendocrine control of hair follicle melanogenesis by locally synthesized factors including adrenocorticotrophic hormone, thyrotrophin-releasing hormone, thyroid hormone, α-melanocyte stimulating hormone, and β-endorphin, melanocortin has been also suggested to trigger or progress graying2122232425262728. Clinical interventions seek to modulate neuroendocrine factor-mediated control of melanogenesis and human melanocyte biology, to prevent or reverse graying29.
Interestingly, graying is sometimes reversed even in the elderly. For example, some non-pigmented (white) hairs begin to produce melanin once more after exposure to radiation or cytokines, becoming proximally pigmented. One explanation is that melanocytes negative for 3,4-dihydroxyphenylalanine and most melanocyte-specific makers remain in the ORSs of white hairs and begin to produce pigment after stimulation3031.
Thus, European researchers, including Paus and Tobin, considered that graying is caused by depletion or dysfunction of melanin-producing melanocytes located in the hair matrix near the dermal papillae of hair follicles. However, amelanogenic melanocytes are also present in the ORSs. Such melanocytes may be able to migrate and differentiate into melanogenic melanocytes, when certain stimuli are applied. Many researchers have sought to identify such re-activating molecules recently.
Nishimura et al. focused on the role played by melanocyte stem cells (MSCs) in the hair bulge, as opposed to follicular melanocytes, reporting that graying is caused by defective MSC self-maintenance. Such maintenance requires BCL-2 and the micro-ophthalmia-associated transcriptional factor (MITF). Also, transforming growth factor-β (TGF-β) and collagen XVII (Col17A1) are involved in regulation of MSC maintenance323334.
Col17A1, a hemidesmosomal transmembrane collagen, is highly expressed in follicular stem cells. MSCs of the hair bulge do not express Col17A1, but premature graying and hair loss were evident in mice lacking Col17a1. Expression of COL17A1 in basal keratinoctyes and follicular stem cells of COL17a1-deficient mice blocked premature MSC differentiation and restored TGF-β signaling. Hair follicular stem cells in the bulge-sub-bulge area provide a functional niche for the MSCs35.
Defective MSC self-maintenance attributable to a Bcl-2 deficiency triggers selective MSC apoptosis, but not apoptosis of bulbar melanocytes. Aging or injury to MSCs induces ectopic pigmentation and/or differentiation within the hair bulge niche, accelerated by Mitf, a key transcriptional regulator of melanogenesis. The roles played by stem cell apoptosis and ectopic differentiation remain unclear; both may contribute to MSC loss to a similar extent32. However, the work of Fisher and Nishimura is essentially mouse-based. Further study should be done in humans to unveil hair graying.
Graying is associated with age-related defects in MSC maintenance. Irreversible DNA damage caused by genotoxic stressors such as ionizing radiation interrupts MSC comeback in mice. MSC depletion via differentiation or apoptosis triggers ‘irreversible’ graying. A defect in the ataxia-telangiectasia mutated (ATM) gene, a kinase serving to transduce the DNA damage response, induces ectopic MSC differentiation. ATM action is important for the maintenance of MSC stemness36.
Recent work has focused principally on MSCs of the hair bulge, not on bulbar melanocytes. The B-Raf and C-Raf kinases that play critical roles in melanoma development are required for MSC maintenance, but not for melanocyte lineage development. Hair graying was evident in a double-Raf knockout mouse37. Human amelanotic melanocytes may be considered to be analogous to the MSCs of mice. The the molecules including BCL-2, MITF, B-Raf, B-Raf, TGF-β, Pax3, Wnt, and Notch have been suggested to be useful markers of human MSCs. However, no specific marker of MSCs has yet been identified, because it is difficult to perform genetic studies in humans38.
We found that Sox10, Pax3, and MITF-M (specific markers of MSCs or melanocytes) were absent from the bulbs of white, but not black, hairs in humans. Thus, both melanocytes and MSCs or amelanotic melanocytes were absent from the bulbs, suggesting that graying may be caused by a depletion of MSC migrating into the hair bulb39.
Earlier, we presented data supporting the idea that graying was attributable to MSC depletion in the hair bulge39. We found the difference in genes/proteins expression associated with thickness and mechanical property, e.g., keratins (KRTs) and keratin-associated proteins (KRTAP), between pigmented and non-pigmented (white) hairs. Interestingly, Van Neste40 found that non-pigmented (white) hair follicles grow significantly more rapidly than pigmented hair follicles in organ culture. Clinically, the growth and thickness of non-pigmented follicles are significantly greater than those of pigmented follicles41.
Our previous studies showed that a few long hairs on the eyebrow are white, while short hairs on the eyebrows are usually black41. There is no report that hair graying may be more severe in farmers than office workers. These may suggest that external stress such as ultraviolet light may be less important in terms of graying than was formerly thought. Thus, we propose that graying is attributable principally to active hair growth.
As expected, we found that KRTs, including KRT6, KRT14/16, and KRT25, were expressed more extensively in non-pigmented (white) than pigmented (black) hairs. Many isoforms of KRTAP were upregulated in white compared with black hairs4243. The expression level of fibroblast growth factor 5 (FGF5), an upstream inhibitor of KRT and KRTAP synthesis, was reduced in white compared with black hairs4445. In contrast, the expression level of FGF7, an upstream stimulator of KRT and KRPAP synthesis, was increased in white compared with black hairs4647. In general, genes and proteins associated with active hair growth are expressed more prominently in white (non-pigmented) than black (pigmented) hairs. Active hair growth, combination of thickness and rate of growth, may result from upregulation of metabolic activity and cell divisions, which may lead to ROS production and MSC depletion in hair follicles. Thus, we suggest that graying is associated with active hair growth41; however, confirmation is required.
As discussed above, non-pigmented (white) hairs grow more rapidly to form thicker bundles, than do pigmented hairs, as a consequence of the absence of melanin in bulbar keratinocytes. Many researchers believe that the active growth of non-pigmented (white) hairs simply reflects the absence of melanin in such hairs. However, there is no evidence that non-pigmented hairs grow more rapidly or thickly than pigmented hairs in vivo.
Animal models of active hair growth such as the FGF5-deficient mouse48, which has longer body hairs than the wild-type mouse, may prove our theory. If we are correct, gray hairs may appear earlier in aging FGF5-deficient mice than in age-matched wild-type mice. Clinically, long-term follow-up of changes in the longer and thicker ‘pigmented’ hairs of the eyebrow, which eventually become non-pigmented (white), may also validate our theory.
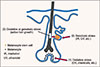
Together, our data suggest that graying may be attributable to active hair growth. Thus, graying may be delayed by suppressing such growth. This could be much more effective than seeking to revert white (non-pigmented) hairs to pigmented hairs, as white hairs containing MSCs or amelanogenic melanocytes are rare in the human scalp. The summary diagram for three theories for the mechanism of hair graying is shown in Fig.1.
Hair graying is closely associated with MSC depletion in the hair bulge. Although re-activation of remaining MSCs or amelanogenic melanocytes may sometimes restore pigmentation, hair bulb melanocyte dysfunction may contribute temporarily to graying. The questions of whether melanin in hair follicles suppress hair growth and whether the principal cause of MSC depletion during graying is external (ultraviolet light, etc.) or internal (active hair growth), remain open.
Figures and Tables
Fig. 1
Hair graying may be caused by (1) depletion or dysfunction of melanocytes producing melanin in the hair matrix near the dermal papilla of the hair follicle (the theory of Tobin and Paus), (2) defective hair bulge MSC self-maintenance via genotoxic stress (the theory of Fisher and Nishimura), and/or (3) oxidative or genotoxic stress associated with active hair growth (the theory of Lee et al.).
ACKNOWLEDGMENT
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A01060018).
References
2. Panhard S, Lozano I, Loussouarn G. Greying of the human hair: a worldwide survey, revisiting the ‘50’ rule of thumb. Br J Dermatol. 2012; 167:865–873.

3. Lison M, Kornbrut B, Feinstein A, Hiss Y, Boichis H, Goodman RM. Progressive spastic paraparesis, vitiligo, premature graying, and distinct facial appearance: a new genetic syndrome in 3 sibs. Am J Med Genet. 1981; 9:351–357.

4. Shin H, Ryu HH, Yoon J, Jo S, Jang S, Choi M, et al. Association of premature hair graying with family history, smoking, and obesity: a cross-sectional study. J Am Acad Dermatol. 2015; 72:321–327.

5. Tobin DJ, Paus R. Graying: gerontobiology of the hair follicle pigmentary unit. Exp Gerontol. 2001; 36:29–54.

6. Trüeb RM, Tobin DJ. Aging hair. Hedelberg: Springer;2010. p. 77–89.
7. Doubaj Y, De Sandre-Giovannoli A, Vera EV, Navarro CL, Elalaoui SC, Tajir M, et al. An inherited LMNA gene mutation in atypical Progeria syndrome. Am J Med Genet A. 2012; 158A:2881–2887.
8. Di Giacomo TB, Valente NY, Nico MM. Chloroquine - induced hair depigmentation. Lupus. 2009; 18:264–266.
9. Donovan JC, Price VH. Images in clinical medicine. Chloroquine-induced hair hypopigmentation. N Engl J Med. 2010; 363:372.
10. Chakrabarty S, Krishnappa PG, Gowda DG, Hiremath J. Factors associated with premature hair graying in a young Indian population. Int J Trichology. 2016; 8:11–14.

11. Seiberg M. Age-induced hair greying - the multiple effects of oxidative stress. Int J Cosmet Sci. 2013; 35:532–538.

12. Commo S, Gaillard O, Bernard BA. Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. Br J Dermatol. 2004; 150:435–443.

13. Arck PC, Overall R, Spatz K, Liezman C, Handjiski B, Klapp BF, et al. Towards a “free radical theory of graying”: melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006; 20:1567–1569.

14. Wood JM, Decker H, Hartmann H, Chavan B, Rokos H, Spencer JD, et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009; 23:2065–2075.

15. Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005; 124:13–21.

16. Seiberg M. Keratinocyte-melanocyte interactions during melanosome transfer. Pigment Cell Res. 2001; 14:236–242.

17. Joshi PG, Nair N, Begum G, Joshi NB, Sinkar VP, Vora S. Melanocyte-keratinocyte interaction induces calcium signalling and melanin transfer to keratinocytes. Pigment Cell Res. 2007; 20:380–384.

18. Nagl W. Different growth rates of pigmented and white hair in the beard: differentiation vs. proliferation? Br J Dermatol. 1995; 132:94–97.

19. Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993; 101:1 Suppl. 90S–97S.

20. Slominski A, Paus R, Schadendorf D. Melanocytes as “sensory” and regulatory cells in the epidermis. J Theor Biol. 1993; 164:103–120.

22. Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005; 19:1332–1334.

23. Kauser S, Thody AJ, Schallreuter KU, Gummer CL, Tobin DJ. A fully functional proopiomelanocortin/melanocortin-1 receptor system regulates the differentiation of human scalp hair follicle melanocytes. Endocrinology. 2005; 146:532–543.

24. Kauser S, Slominski A, Wei ET, Tobin DJ. Modulation of the human hair follicle pigmentary unit by corticotropin-releasing hormone and urocortin peptides. FASEB J. 2006; 20:882–895.

25. Tobin DJ, Kauser S. Hair melanocytes as neuro-endocrine sensors--pigments for our imagination. Mol Cell Endocrinol. 2005; 243:1–11.

26. Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009; 19:R132–R142.

27. Meyer KC, Brzoska T, Abels C, Paus R. The alpha-melanocyte stimulating hormone-related tripeptide K(D)PT stimulates human hair follicle pigmentation in situ under proinflammatory conditions. Br J Dermatol. 2009; 160:433–437.

28. Paus R, Arck P. Neuroendocrine perspectives in alopecia areata: does stress play a role? J Invest Dermatol. 2009; 129:1324–1326.

29. Paus R. A neuroendocrinological perspective on human hair follicle pigmentation. Pigment Cell Melanoma Res. 2011; 24:89–106.

30. Takada K, Sugiyama K, Yamamoto I, Oba K, Takeuchi T. Presence of amelanotic melanocytes within the outer root sheath in senile white hair. J Invest Dermatol. 1992; 99:629–633.

31. Horikawa T, Norris DA, Johnson TW, Zekman T, Dunscomb N, Bennion SD, et al. DOPA-negative melanocytes in the outer root sheath of human hair follicles express premelanosomal antigens but not a melanosomal antigen or the melanosome-associated glycoproteins tyrosinase, TRP-1, and TRP-2. J Invest Dermatol. 1996; 106:28–35.

32. Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005; 307:720–724.

33. Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y, et al. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell. 2010; 6:130–140.

34. Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011; 24:401–410.

35. Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011; 8:177–187.

36. Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, Wakayama T, et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009; 137:1088–1099.

37. Valluet A, Druillennec S, Barbotin C, Dorard C, Monsoro-Burq AH, Larcher M, et al. B-Raf and C-Raf are required for melanocyte stem cell self-maintenance. Cell Rep. 2012; 2:774–780.

38. Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014; 14:1569–1579.

39. Choi YJ, Yoon TJ, Lee YH. Changing expression of the genes related to human hair graying. Eur J Dermatol. 2008; 18:397–399.
40. Van Neste D. Thickness, medullation and growth rate of female scalp hair are subject to significant variation according to pigmentation and scalp location during ageing. Eur J Dermatol. 2004; 14:28–32.
41. Choi HI, Choi GI, Kim EK, Choi YJ, Sohn KC, Lee Y, et al. Hair greying is associated with active hair growth. Br J Dermatol. 2011; 165:1183–1189.

42. Shimomura Y, Aoki N, Rogers MA, Langbein L, Schweizer J, Ito M. Characterization of human keratin-associated protein 1 family members. J Investig Dermatol Symp Proc. 2003; 8:96–99.

43. Schweizer J, Langbein L, Rogers MA, Winter H. Hair follicle-specific keratins and their diseases. Exp Cell Res. 2007; 313:2010–2020.

44. Ota Y, Saitoh Y, Suzuki S, Ozawa K, Kawano M, Imamura T. Fibroblast growth factor 5 inhibits hair growth by blocking dermal papilla cell activation. Biochem Biophys Res Commun. 2002; 290:169–176.

45. Drögemüller C, Rüfenacht S, Wichert B, Leeb T. Mutations within the FGF5 gene are associated with hair length in cats. Anim Genet. 2007; 38:218–221.

46. Danilenko DM, Ring BD, Yanagihara D, Benson W, Wiemann B, Starnes CO, et al. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am J Pathol. 1995; 147:145–154.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download