Abstract
Background
Xanthium stramarium (XAS) and Psoralea corylifolia (PSC), phototoxic oriental medicinal plants, has been used in traditional medicines in Asian countries.
Objective
The effects of highly purified XAS or PSC extract combined with ultraviolet A1 (UVA1) irradiation on cell proliferation and transforming growth factor-beta1 (TGF-β1) expression of the keloid fibroblast were being investigated to define potential therapeutic uses for keloid treatments.
Methods
The keloid fibroblasts were treated with XAS or PSC alone or in the combination with UVA1 irradiation. The cell viability, apoptosis, and expression of TGF-β1 and collagen I were investigated.
Keloid is a pathological response of wound healing to cutaneous injury in genetically susceptible individuals. It is characterized by fibroblastic proliferations and accumulations of collagen1. Although the pathogenesis of keloid is still unclear, it has been known that keloid fibroblasts, when compared with normal fibroblasts, have lower rates of apoptosis1. In addition, these cells overproduces type I collagen and expresses higher levels of cytokines and growth factors, which influences proliferation and collagen synthesis by fibroblasts. Transforming growth factor (TGF)-β1 is well known as a key fibrogenic cytokine promoting collagen production and tissue fibrosis in keloids. Therefore, controlling fibroblast apoptosis and fibrogenic action of TGF-β1 are of therapeutic interests1-3. The use of photochemotherapy has been reported to be beneficial for the treatment of keloid and morphea4. It was suggested that ultraviolet (UV) irradiation suppresses cellular immunity and also has antifibrotic effects5. Psoralen and ultraviolet A (PUVA) therapy including oral-, topical- and bath-PUVA was effective in localized scleroderma6,7. However, the treatment effects of photochemotherapy for keloid and morphea are still challenging. Moreover, the well-known photosensitizer, psoralen is not commercially available in Korea.
Xanthium stramarium (XAS) has been used for the treatment of various kinds of disorders such as skin fungal infection, rhinitis, and eczema, and Psoralea corylifolia (PSC) also has been used for asthma, cough, nephritis, vitiligo, and calvities in traditional medicines of Asian countries8,9. Interest has been brought based on its putative beneficial pharmacological effects, of antioxidant and anti-carcinogenic effects through inhibiting cell proliferation and increasing apoptosis8,9. More interestingly, there have also been reports that XAS and PSC, which have properties of phototoxicity could be considered as alternative photosensitizing agents for photochemotherapy10. Plants showed significant absorption peaks at the ultraviolet A (UVA) or blue regions of visible light and strong fluorescence emissions at red light and phototoxic reactions in in vitro and in vivo mice10. Specifically, the phototoxic properties of XAS and PSC have been shown to be stronger than those of psoralen for phototherapy in dermatological fields8.
Therefore, in this study, we investigated the cellular effects of extracted XAS and PSC in combination with UVA1 irradiation on keloid fibroblasts and sought laboratory supports for opening the possibility of clinical treatments. The effects of the combination treatment on cell growth and TGF-β1 and collagen expressions in keloid fibroblasts were investigated.
The seeds of cocklebur (XAS L.) and matured seeds of PSC were extracted with methanol. The mixture was filtered over filter paper (Whatman No. 1; Whatman International Ltd., Maidstone, UK). The filtrate was concentrated using a rotatory evaporator (Eyela N-2100, Tokyo Rikakikai Co, Ltd., Tokyo, Japan). The portion of a sticky solid (0.5 g) was chromatographed over prep silica gel (TLC Silica gel 60 F254; Merck KGaA, Darmstadt, Germany) using methanol eluent. The band emitting a red fluorescence under a UV illumination was separated and extracted with methanol. The solvent was removed using rotatory evaporator under a reduced pressure to deliver white solids (1.5 mg XAS and 1.2 mg PSC).
Keloid tissues were extracted from 5 patients (2 men and 3 women as a mean age of 25 years), who gave written informed consent for use of their excised tissue for academic research. Fibroblasts derived from keloid tissues were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 1% antibiotic/antimycotic solution (all from Gibco/BRL, Grand Island, NY, USA). All cultures were maintained at 37℃ in humidified (5% CO2) incubators. The cells were sub-cultured at 80~90% confluence using trypsin. Only cells from passage two to seven were analyzed in this study. Normal human fibroblasts from foreskin were cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum and 1% antibiotic/antimycotic solution.
Keloid fibroblasts (5×104 cells) were cultured on 60φ plates for 24 hours. After washing with phosphate buffer saline (PBS), the cells were cultured with RPMI-1640 medium without serum and were treated with XAS or PSC. After 1 hour of incubation, the cells were exposed to the UVA1 using a UVA1 Sellamed System (Sellas, Gevelsberg, Germany) and were maintained for 24 hours.
The cells (7×103 cells) were cultured on 96-well culture plates and treated with XAS (1~1,000 µg/ml) or PSC (1~100 µg/ml) for 25 hours alone or with combination with UVA1 irradiation and harvested. The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) solution was added to each well and the plates were incubated at 37℃ for 4 hours. The resulting formazan crystals were dissolved in dimethylsulphoxide and optical density was measured using the microplate reader (Bio-Tek, Winooski, VT, USA) at 560 nm.
Apoptosis was determined by the TUNEL method using an in situ cell detection kit (Roche Molecular Biochemicals, Mannheim, Germany). The Keloid fibroblasts were treated with XAS (50 µg/ml) or PSC (10 µg/ml) alone or in combination with UVA1 irradiation. The cells were washed with PBS and fixed in 4% paraformaldehyde. The cells were then incubated with 50 µl TUNEL reaction mixture (TdT and fluorescin-dUTP) at 37℃ for 60 minutes and 5 mg/ml Hoechst 33258 (Sigma Chemical Co., St. Louis, MO, USA) for 5 minutes. The stained cells were analyzed under a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
The cells were seed at 5×104 cells into 60φ plates and treated with XAS (50 µg/ml), or PSC (10 µg/ml) alone or in combination with UVA1 (30 J/cm2). After harvesting, the supernatants were obtained by filtering with a 0.22-µm pore-size membrane filter. The levels of TGF-β1 were measured by ELISA using the manufacturer's protocol (R&D Systems Inc., Minneapolis, MN, USA).
Cells were collected and lysed in the lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1% NP 40, 1% sodium dodecyl sulfate [SDS], 0.5% deoxycholic acid and 1 mM EDTA, protease and phosphatase inhibitors). Cells lysates were subjected to SDS-polyacrylamide gel electrophoresis and transferred onto polyvinyldene difluoride (PVDF) membrane. The protein blots were then incubated with antibodies and detection of antibody binding was performed using enhanced chemiluminescence (Amersham Biosciences, Buckinghamshire, UK).
Cells were seeded in 60φ culture dish at 5×104 cells. Total cellular RNA was extracted using RNeasy mini kit (Qiagen Inc., Valencia, CA, USA), and the cDNA was obtained using SuperScript TM III reverse transcriptase kit (Invitrogen, Carlsbad, CA, USA). Expression of TGF-β1, Collagen I and GAPDH were measured using semiquantitative PCR. The oligonucleotide primers for PCR were synthesized by Bioneer and were as follows: TGF-β1, sense oligonucleotide 5'-CCGACTACTACGCCAAGGA-3' and antisense oligonucleotide 5'-AGTGAACCCGTTGATG TCCA-3'; Collagen I, sense oligonucleotide 5'-AGCATGA CCGA TGGATTCCA-3' and antisense oligonucleotide 5'-GGAGGGAGTTTACAGGAAGCA; GAPDH, sense oligonuleotide 5'-GAGTACGTCGTGGAGTCCA-3' and antisense oligo nucleotide 5'-ATGGCATGGACTGTGGTCA-3'. PCR amplification was performed with a GeneAmp PCR system 2700 (Applied Biosystems, Foster City, CA, USA) under the following conditions: TGF-β1, Collagen I: 30 cycle at 94 for 30 s, 55 for 30 s, and 72 for 30 s. The PCR products were electrophoresed on 1% agarose gels.
First, we have determined the appropriate dose of UVA1, and working concentrations of XAS and PSC for the study. The keloid fibroblasts were irradiated with variable intensity of UVA1 and the cell viability was monitored using MTT proliferation assay. To investigate combination effects, 30 J/cm2 of UVA1 was determined to be minimal-cytotoxic to the keloid fibroblasts. It was noted that the same dose of UVA1 was extremely cytotoxic to the normal fibroblasts as compared to the keloid fibroblasts (Fig. 1A). Next, dose titration studies of XAS or PSC were performed. Keloid fibroblasts were incubated with XAS (1, 50, 100, 200, 100, and 1,000 µg/ml) or PSC (1, 5, 10, 50, and 100 µg/ml). As shown in Fig. 1, keloid fibroblasts treated with XAS (50~1,000 µg/ml) or PSC (5~100 µg/ml) in combination with UVA1 irradiation showed a significant inhibition of cell growth (Fig. 1B~D). Treatment with XAS (50 µg/ml) or PSC (10 µg/ml) combined with UVA1 irradiation resulted in moderate cell cytotoxicity. Therefore, a working concentration of XAS (50 µg/ml) and PSC (10 µg/ml) combined with 30 J/cm2 UVA1 was chosen for all subsequent studies. It was noted that greater than XAS (200 µg/ml) or PSC (50 µg/ml) itself showed significant cell cytotoxicity. Interestingly, the inhibitory effects of the XAS and PSC in combination with UVA1 were better than the effects of psoralen in combination with UVA1 (Fig. 1E). To further investigate whether the inhibitory effects of XAS or PSC on proliferation of keloid fibroblasts is dependent with cell apoptosis, we stained cells using Hoechst 33258 and TUNEL after XAS or PSC treatment in combination with UVA1 irradiation. As seen in Fig. 1F, XAS (50 µg/ml) or PSC (10 µg/ml) in combination with UVA1 irradiation increased the number of TUNEL-positive cells. These results suggest that the reduced cell viability by XAS or PSC treatment in keloid fibroblasts appeared to occur dependently of apoptosis.
TGF-β1 is an essential profibrotic cytokine for collagen synthesis, and administration of TGF-β1 resulted in a dramatic increase in intracellular collagen I levels in keloid fibroblasts11. To investigate the role of the treatment on the TGF-β1 and collagen expressions in keloid firboblasts, the ELISA analysis was first carried out (Fig. 2A). As a result, the XAS (50 µg/ml) or PSC (10 µg/ml) treatment in combination with UVA1 irradiation inhibited the TGF-β1 expression in the keloid fibroblasts. The inhibitory effects of the XAS and PSC in combination with UVA1 were better than the effects of psoralen in combination with UVA1. Reverse transcription-polymerase chain reaction study showed that XAS (50 µg/ml) or PSC (10 µg/ml) reduced collagen I and TGF-β1 mRNA expressions (Fig. 2B). Western blotting further confirmed the inhibitory actions of XAS or PSC in combination with UVA1 irradiation on collagen I protein expression (Fig. 2C). These results suggest that XAC or PSC in combined with UVA1 irradiation also regulate collagen production by TGF-β1 in the keloid fibroblasts as well as suppressed keloid fibroblast viabilities. Interestingly, XAS or PSC itself showed inhibitory actions on TGF-β1 expression.
The present study showed that XAS and PSC in combination with UVA1 could suppress proliferation and induce apoptosis of keloid fibroblasts. It was also shown that XAS and PSC in combination with UVA1 cause inhibition of TGF-β1 and collagen expression of the cells. At the concentrations without cell cytotoxicity, the XAS or PSC synergize the UVA1 effects on keloid fibroblasts which suggest the phototoxic potential of the plants. The phototoxic properties of XAS and PSC have been shown to be stronger than those of psoralen8. We also found the inhibitory effects of the XAS and PSC in combination with UVA1 was better than the effects of psoralen in combination with UVA1 (Fig. 1E, Fig. 2A).
Interestingly, the XAS or PSC itself showed inhibitory actions on TGF-β1 and collagen expressions. These beneficial effects seem to be related to their putative pharmacological effects including antioxidant and anti-inflammatory actions8,9. Flavonoids are found in well-known topical scar creams, where quercetin has been shown to inhibit fibroblast proliferation, collagen production and contraction of keloid. XAS are known for containing sesquiterpene lactones termed xanthanolides, which are responsible for most of the biological activities of Xanthium species9. It has been proposed that XAS has anti-inflammatory and antioxidant activities such as the inhibition of cyclooxygenase 2, the effect on NF-κB and others. PSC contains psoralen and isopsoralen which was suggested to contribute to antioxidant effect as well as phototoxic effects of the plant8.
PUVA therapy is thought to enhance the gene expression of collagenase12, reduce gene expression of type I and type III collagens13, and decrease the number of collagen cross-links14. Given the in vitro work regarding the effect of UVA1 on stimulated collagenase production by fibroblasts and the advantages of UVA1 including deeper penetration and relative safety from carcinogenicity15, the present findings may open up the possibility of testing these plants in combination with UVA1 irradiation for clinical keloid treatments. However, the precise mechanisms of the effects of XAC or PSC and the combination with UV irradiation on the cellular proliferation of keloid fibroblast still needs to be further explored. Further chemical isolation and characterization of the active compounds from these plants extract are also necessary.
Figures and Tables
Fig. 1
Xanthium stramarium (XAS) and Psoralea corylifolia (PSC) in combination with ultraviolet A1 (UVA1) irradiation suppressed cell growth of keloid fibroblasts via apoptosis. (A) Keloid fibroblasts and normal fibroblasts were irradiated with 30 J/cm2 of UVA1. The cell viability was monitored using MTT proliferation assays. UVA1 irradiation showed minimal-cytotoxic to the keloid fibroblasts. On the other hands, the same dose of UVA1 was extremely cytotoxic to the normal fibroblasts compared to the keloid fibroblasts. *p<0.001. Keloid fibroblasts were treated with various concentrations of XAS (B) or PSC (C) alone or in combination with UVA1 irradiation (30 J/cm2). Treatment with XAS (50 µg/ml) or PSC (10 µg/ml) in combination with UVA1 irradiation resulted in moderate cell cytotoxicity. Data represent the mean±standard deviation of independent three experiments. #p<0.001, as compared with the control, *p<0.001, when compared with the control treated with UVA1 irradiation. (D) The light microscopic images of the cells treated with XAS (50 µg/ml) or PSC (10 µg/ml) in combination with UVA1 irradiation (×200). (E) The inhibitory effects of the XAS and PSC in combination with UVA1 were better than the effect of psoralen in combination with UVA1. (F) Apoptotic cells were detected by terminal deoxynucleotidyl transferase-mediated dUTP biotin nick end labeling (TUNEL) assay and Hoechst 33258 staining (×200). XAS (50 µg/ml) or PSC (10 µg/ml) in combination with UVA1 irradiation increased the number of TUNEL-positive cells. The results shown here were reproducible in independent three experiments.
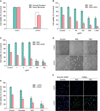
Fig. 2
Xanthium stramarium (XAS) and Psoralea corylifolia (PSC) in combination with ultraviolet A1 (UVA1) irradiation suppressed transforming growth factor-beta1 (TGF-β1) expression and collagen I production in keloid fibroblasts. (A) The expression of TGF-β1 was analyzed by Enzyme-linked immunosorbent assay (ELISA). XAS (50 µg/ml) or PSC (10 µg/ml) treatment in combination with UVA1 irradiation inhibited the TGF-β1 expression in the keloid fibroblasts. The inhibitory effects of the XAS and PSC in combination with UVA1 were better than the effects of psoralen in combination with UVA1. #p<0.001, as compared with the control, *p<0.001, when compared with the control treated with UVA1 irradiation. The values represent mean±standard deviation of independent three experiments. (B) Reverse transcription-polymerase chain reaction (RT-PCR) study also showed that XAS (50 µg/ml) or PSC (10 µg/ml) reduced collagen I and TGF-β1 expression. (C) Western blotting further confirmed the inhibitory actions of XAS or PSC in combination with UVA1 irradiation on collagen I expression.

ACKNOWLEDGMENT
This work was supported by grants of the Korean Health Technology R&D project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A100179).
References
1. Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011; 17:113–125.

2. Diao JS, Xia WS, Yi CG, Wang YM, Li B, Xia W, et al. Trichostatin A inhibits collagen synthesis and induces apoptosis in keloid fibroblasts. Arch Dermatol Res. 2011; 303:573–580.

3. Huang L, Wong YP, Cai YJ, Lung I, Leung CS, Burd A. Low-dose 5-fluorouracil induces cell cycle G2 arrest and apoptosis in keloid fibroblasts. Br J Dermatol. 2010; 163:1181–1185.

4. Hannuksela-Svahn A, Grandal OJ, Thorstensen T, Christensen OB. UVA1 for treatment of keloids. Acta Derm Venereol. 1999; 79:490.

5. Sunderkötter C, Kuhn A, Hunzelmann N, Beissert S. Phototherapy: a promising treatment option for skin sclerosis in scleroderma? Rheumatology (Oxford). 2006; 45:Suppl 3. iii52–iii54.
6. Morison WL. Psoralen UVA therapy for linear and generalized morphea. J Am Acad Dermatol. 1997; 37:657–659.

7. Kerscher M, Meurer M, Sander C, Volkenandt M, Lehmann P, Plewig G, et al. PUVA bath photochemotherapy for localized scleroderma. Evaluation of 17 consecutive patients. Arch Dermatol. 1996; 132:1280–1282.

8. Wang Y, Hong C, Zhou C, Xu D, Qu HB. Screening antitumor compounds psoralen and isopsoralen from Psoralea corylifolia L. Seeds. Evid Based Complement Alternat Med. 2011; 2011:363052.
9. Nibret E, Youns M, Krauth-Siegel RL, Wink M. Biological activities of xanthatin from Xanthium strumarium leaves. Phytother Res. 2011; 25:1883–1890.

10. Bark KM, Heo EP, Han KD, Kim MB, Lee ST, Gil EM, et al. Evaluation of the phototoxic potential of plants used in oriental medicine. J Ethnopharmacol. 2010; 127:11–18.

11. Li J, Cao J, Li M, Yu Y, Yang Y, Xiao X, et al. Collagen triple helix repeat containing-1 inhibits transforming growth factor-b1-induced collagen type I expression in keloid. Br J Dermatol. 2011; 164:1030–1036.

12. Scharffetter K, Wlaschek M, Hogg A, Bolsen K, Schothorst A, Goerz G, et al. UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch Dermatol Res. 1991; 283:506–511.

13. Kitamura Y, Namikawa H, Hayashi S, Yoshida T, Suzuki T, Hamasaki Y, et al. Effects of UVA irradiation following treatment with 8-methoxypsoralen on type I and type III collagen synthesis in normal and scleroderma fibroblast cultures. Arch Dermatol Res. 2009; 301:507–513.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download