Abstract
The porcine epidemic diarrhea virus (PEDV) has recently been shown to cause huge economic losses in the global pork industry. Our results demonstrated that the extract dose-dependently inhibited the replication of PEDV and reduced the visible cytopathic effect (CPE). Treatment with C. heterophylla Fisch extract resulted in marked reduction of PEDV-induced cytokine and chemokine expression. The antiviral activity of C. heterophylla Fisch extract on PEDV replication was found to be primarily exerted at the early stages after infection. Taken together, our data indicate that C. heterophylla Fisch extract may be a good therapeutic agent for use against PEDV and also a potential candidate to be evaluated against other human and animal coronaviruses.
REFERENCES
2). Temeeyasen G, Srijangwad A, Tripipat T, Tipsombatboon P, Piriyapongsa J, Phoolcharoen W, et al. Genetic diversity of ORF3 and spike genes of porcine epidemic diarrhea virus in Thailand. Infect Genet Evol. 2014; 21:205–13.

3). Sun D, Wang X, Wei S, Chen J, Feng L. Epidemiology and vaccine of porcine epidemic diarrhea virus in China: a mini-review. J Vet Med Sci. 2016; 78:355–63.

4). Jung K, Saif LJ. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet J. 2015; 204:134–43.

5). Gerber PF, Opriessnig T. Detection of immunoglobulin (Ig) A antibodies against porcine epidemic diarrhea virus (PEDV) in fecal and serum samples. MethodsX. 2015; 2:368–73.

6). Kweon CH, Kwon BJ, Lee JG, Kwon GO, Kang YB. Derivation of attenuated porcine epidemic diarrhea virus (PEDV) as vaccine candidate. Vaccine. 1999; 17:2546–53.

7). Song DS, Yang JS, Oh JS, Han JH, Park BK. Differentiation of a Vero cell adapted porcine epidemic diarrhea virus from Korean field strains by restriction fragment length polymorphism analysis of ORF 3. Vaccine. 2003; 21:1833–42.

8). Shen H, Zhang C, Guo P, Liu Z, Zhang J. Effective inhibition of porcine epidemic diarrhea virus by RNA interference in vitro. Virus Genes. 2015; 51:252–9.
9). Järvinen P, Palmé A, Orlando Morales L, Lännenpää M, Keinänen M, Sopanen T, et al. Phylogenetic relationships of Betula species (Betulaceae) based on nuclear ADH and chloroplast matK sequences. Am J Bot. 2004; 91:1834–45.
10). Zhao TT, Zhang J, Liang LS, Ma QH, Chen X, Zong JW, et al. Expression and Functional Analysis of WRKY Transcription Factors in Chinese Wild Hazel, Corylus heterophylla Fisch. PloS One. 2015; 10:e0135315.
11). Plosker GL, Hurst M. Paclitaxel: a pharmacoeconomic review of its use in non-small cell lung cancer. Pharmacoeconomics. 2001; 19:1111–34.
12). Kumar S, Mahdi H, Bryant C, Shah JP, Garg G, Munkarah A. Clinical trials and progress with paclitaxel in ovarian cancer. Int J Womens Health. 2010; 2:411–27.

13). Gradishar WJ. Taxanes for the treatment of metastatic breast cancer. Breast Cancer. 2012; 6:159–71.

14). Choi HJ, Kim JH, Lee CH, Ahn YJ, Song JH, Baek SH, et al. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009; 81:77–81.

15). Chiang LC, Chiang W, Liu MC, Lin CC. In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids. J Antimicrob Chemother. 2003; 52:194–8.
16). Briskin DP. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 2000; 124:507–14.

17). Jassim SA, Naji MA. Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol. 2003; 95:412–27.

18). Vlietinck AJ, Vanden Berghe DA. Can ethnopharmacology contribute to the development of antiviral drugs? J Ethnopharmacol. 1991; 32:141–53.

19). Mukhtar M, Arshad M, Ahmad M, Pomerantz RJ, Wigdahl B, Parveen Z. Antiviral potentials of medicinal plants. Virus Res. 2008; 131:111–20.

20). Li Y, Jiang R, Ooi LS, But PP, Ooi VE. Antiviral triterpenoids from the medicinal plant Schefflera heptaphylla. Phytother Res. 2007; 21:466–70.
21). del Barrio G, Parra F. Evaluation of the antiviral activity of an aqueous extract from Phyllanthus orbicularis. J Ethnopharmacol. 2000; 72:317–22.

Figure 1.
The effects of C. heterophylla Fisch extract on PEDV-induced CPE. Morphological assessment of PEDV-infected Vero cells following treatment with C. heterophylla Fisch extract. (A) Non-infected cells; (B) non-infected cells treated with C. heterophylla Fisch extract 10 μg/ml; (C) non-infected cells treated with C. heterophylla Fisch extract 50 μg/ml; (D) PEDV-infected cells; (E) PEDV-infected cells treated with C. heterophylla Fisch extract 10 μg/ml; (F) PEDV-infected cells treated with C. heterophylla Fisch extract 50 μg/ml.
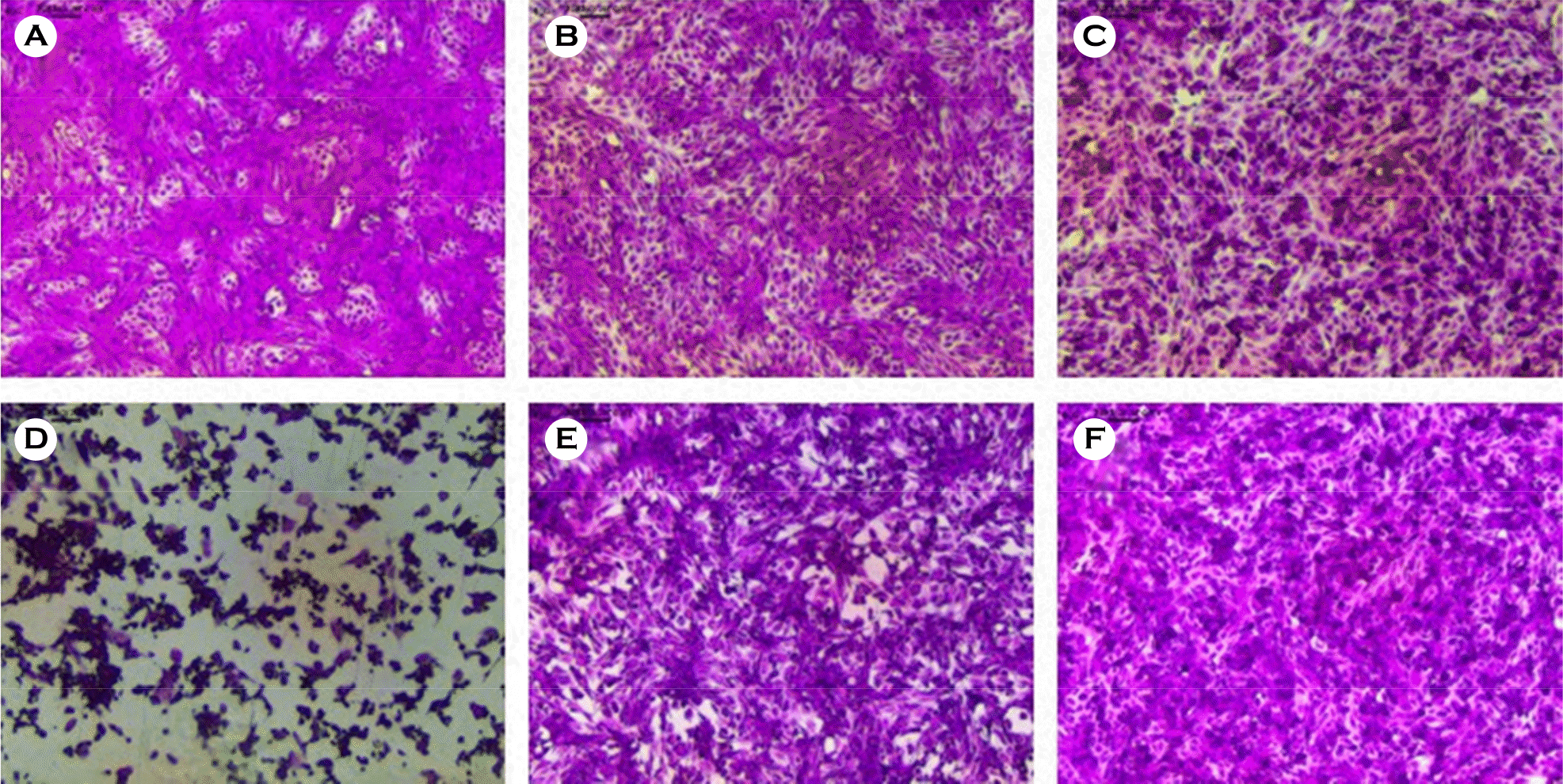
Figure 2.
The antiviral activity of C. heterophylla Fisch against PEDV. (A) Antiviral activity and (B) Cytotoxicity of C. heterophylla Fisch extract against PEDV in Vero cells. Vero cells were infected with PEDV, after which they were treated with the indicated concentrations (0.4, 2, 10, and 50 μg/ml) of C. heterophylla Fisch extract for 48 h. Antiviral activity was investigated using a CPE reduction assay. Data are presented as means ± S.D. from three independent experiments, each carried out in triplicate. ∗∗∗ p < 0.05 (one-way ANOVA followed by the Tukey post hoc test)
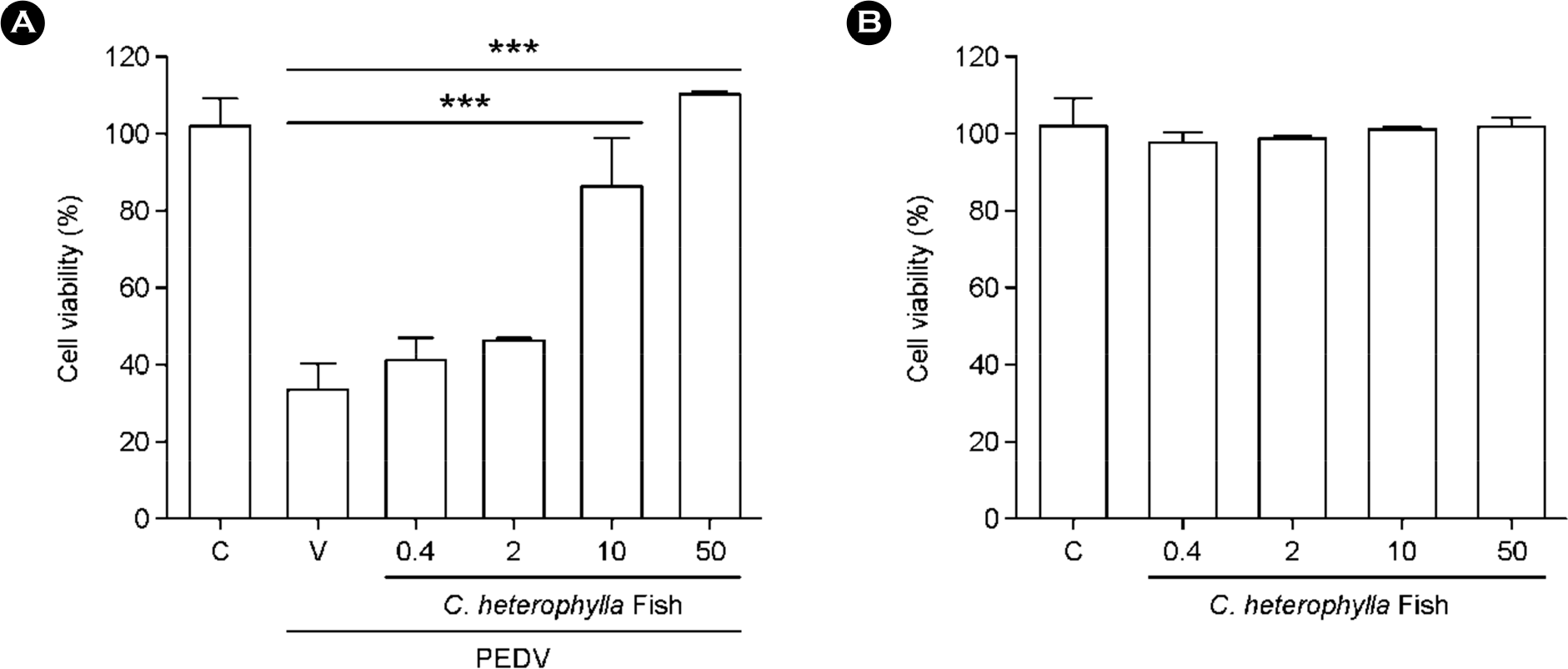
Figure 3.
Cytokine and chemokine gene expression levels reduced by C. heterophylla Fisch extract in Vero cells. Proinflammatory cytokines and chemokines measured in PEDV-infected Vero cells treated with C. heterophylla Fisch extract 6 h after infection.
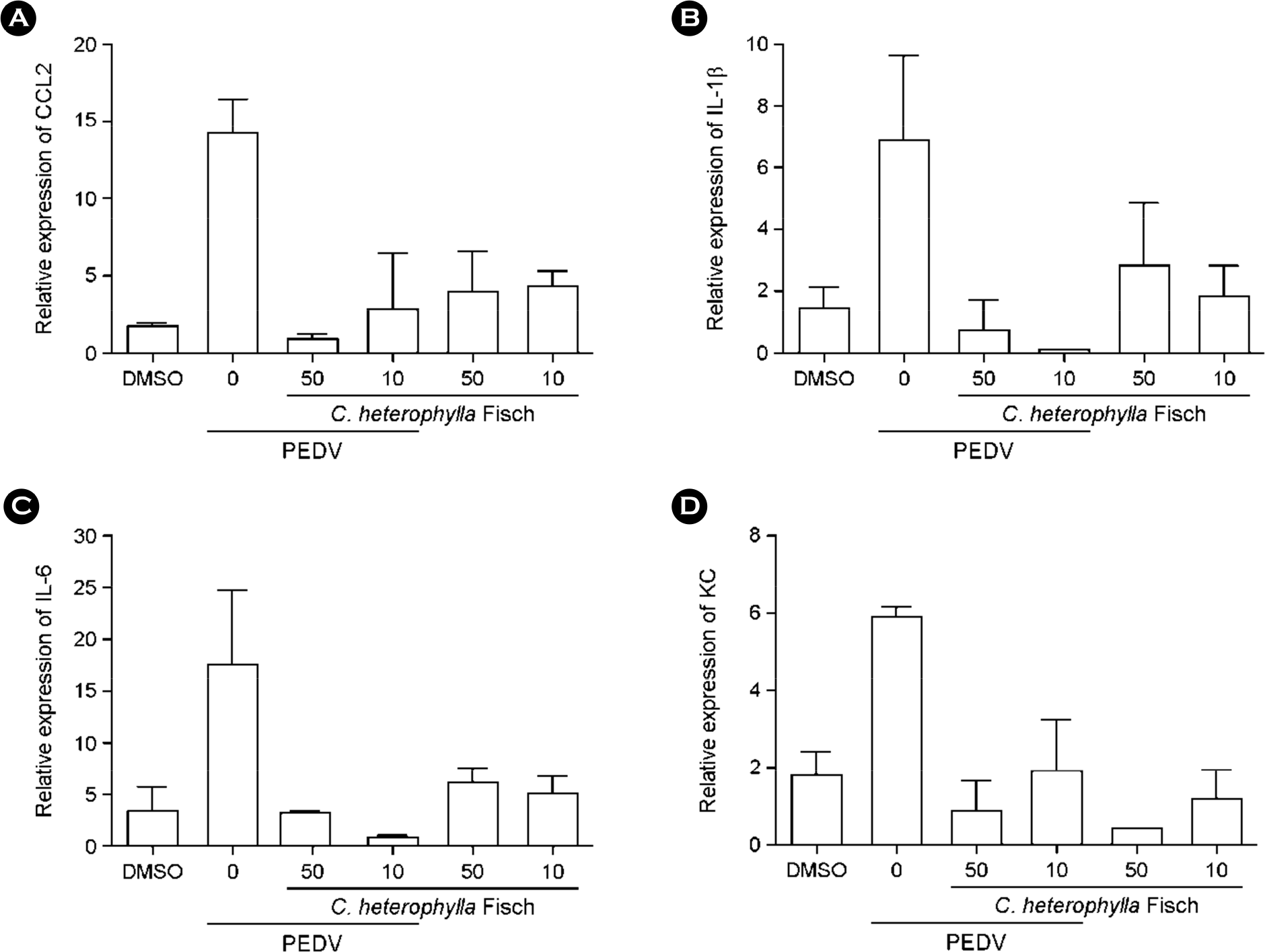
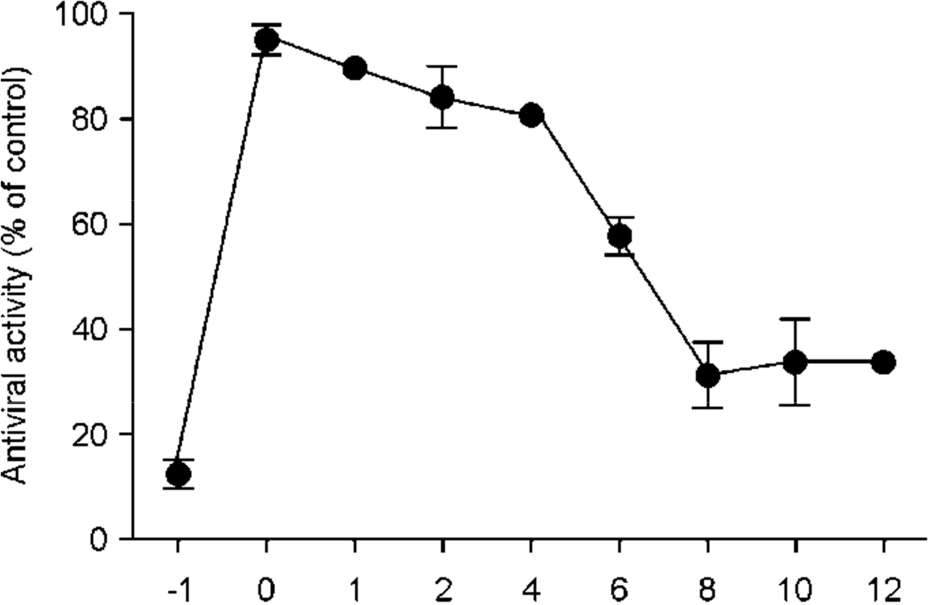
Figure 4.
Time-of-addition effect of C. heterophylla Fisch on PEDV replication in Vero cells. C. heterophylla Fisch extract at 10 μg/ml was added either before (-1 h), during (0 h), or after (1, 2, 4, 6, 8, 10 and 12 h) PEDV infection. After 2 d, inhibition was evaluated using the SRB method and was expressed as the inhibition rate. Each value is the result of mean ± S.D. of three independent experiments.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download