Abstract
Purpose
Neuronal apoptotic events induced by aging and hypoxic/ischemic conditions is an important risk factor in neurodegenerative diseases such as ischemia stroke and Alzheimer's disease. The peel of Citrus sunki Hort. ex Tanaka has long been used as a traditional medicine, based on multiple biological activities including anti-oxidant, anti-inflammation, and anti-obesity. In the current study, we examined the actions of fermented C. sunki peel extract against cobalt chloride (CoCl2)-mediated hypoxic death in human neuroblastoma SH-SY5Y cells.
Methods
Cell viability was measured by trypan blue exclusion. Expression of apoptosis related proteins and release of cytochrome c were detected by western blot. Production of intracellular reactive oxygen species (ROS) and apoptotic morphology were examined using 2',7'-dichlorofluorescin diacetate (DCF-DA) and 4',6-diamidino-2-phenylindole (DAPI) staining.
Results
Exposure to CoCl2, a well-known mimetic agent of hypoxic/ischemic condition, resulted in neuronal cell death via caspase-3 dependent pathway. Extract of fermented C. sunki peel significantly rescued the CoCl2-induced neuronal toxicity with the cell viability and appearance of apoptotic morphology. Cytoprotection with fermented C. sunki peel extract was associated with a decrease in activities of caspase-3 and cleavage of poly (ADP ribose) polymerase (PARP). In addition, increase in the intracellular ROS and release of cytochrome c from mitochondria to the cytosol were inhibited by treatment with extract of fermented C. sunki peel.
Figures and Tables
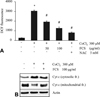
Fig. 1
Induction of caspase-3 mediated cell death. Effects of CoCl2 on the cell viability by trypan blue exclusion (A) and expression of apoptosis-related proteins by western blot analysis (B). Data are represented as mean ± SD of three independent experiments. *p < 0.05 vs control. C: control
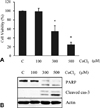
Fig. 2
Protective Effect of fermented C. sunki peel extract on the neurotoxicity (A), activities of caspases and PARP (B), formation of apoptotic body (C) in CoCl2-treated SH-SY5Y cells. Data are presented as mean ± SD of three independent experiments. *p < 0.05 vs control. #p < 0.05 vs CoCl2-treated cells. FCS: Fermented C. sunki peel extract
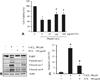
Fig. 3
Effect of fermented C. sunki peel extract on the CoCl2-induced ROS generation (A) and release of mitochondrial cytochrome c to cytosol (B). Data are represented as mean ± SD of three independent experiments. *p < 0.05 vs control. #p < 0.05 vs CoCl2-treated cells. FCS: Fermented C. sunki peel extract, NAC: N-acetyl-cystein, Cyt c: Cytochrome c
References
1. World Health Organization (CH). The top 10 causes of death [Internet]. Geneva: World Health Organization;2013. updated 2014 May. cited 2015 Mar 11. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/.
2. Statistics Korea. 2013 Cause of death statistics [Internet]. Daejeon: Statistics Korea;2014. cited 2014 Sep 23. Available from: http://kostat.go.kr/portal/korea/kor_nw/2/6/2/index.board.
3. Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009; 40(5):e331–e339.

4. Fan X, Jiang Y, Yu Z, Yuan J, Sun X, Xiang S, Lo EH, Wang X. Combination approaches to attenuate hemorrhagic transformation after tPA thrombolytic therapy in patients with poststroke hyperglycemia/ diabetes. Adv Pharmacol. 2014; 71:391–410.
5. Ghosh N, Ghosh R, Bhat ZA, Mandal V, Bachar SC, Nima ND, Sunday OO, Mandal SC. Advances in herbal medicine for treatment of ischemic brain injury. Nat Prod Commun. 2014; 9(7):1045–1055.

6. Lopez MS, Dempsey RJ, Vemuganti R. Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem Int. Forthcoming 2015.

7. Mikami Y, Yamazawa T. Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci. Forthcoming 2015.

8. Yasuda N, Ishii T, Oyama D, Fukuta T, Agato Y, Sato A, Shimizu K, Asai T, Asakawa T, Kan T, Yamada S, Ohizumi Y, Oku N. Neuroprotective effect of nobiletin on cerebral ischemia-reperfusion injury in transient middle cerebral artery-occluded rats. Brain Res. 2014; 1559:46–54.

9. Yabuki Y, Ohizumi Y, Yokosuka A, Mimaki Y, Fukunaga K. Nobiletin treatment improves motor and cognitive deficits seen in MPTP-induced Parkinson model mice. Neuroscience. 2014; 259:126–141.

10. Onozuka H, Nakajima A, Matsuzaki K, Shin RW, Ogino K, Saigusa D, Tetsu N, Yokosuka A, Sashida Y, Mimaki Y, Yamakuni T, Ohizumi Y. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer's disease. J Pharmacol Exp Ther. 2008; 326(3):739–744.
11. Shin HS, Kang SI, Ko HC, Kim HM, Hong YS, Yoon SA, Kim SJ. Anti-inflammatory effect of the immature peel extract of Jinkyool (Citrus sunki Hort. ex Tanaka). Food Sci Biotechnol. 2011; 20(5):1235–1241.

12. Kim YD, Senevirathne M, Koh KS, Jeon YJ, Kim SH. Reactive oxygen species scavenging activity of Jeju native citrus peel during maturation. J Korean Soc Food Sci Nutr. 2009; 38(4):462–469.

13. Moon SW, Kang SH, Jin YJ, Park JG, Lee YD, Lee YK, Park DB, Kim SJ. Fermentation of Citrus unshiu Marc. and functional characteristics of the fermented products. Korean J Food Sci Technol. 2004; 36(4):669–676.
14. Kang SI, Shin HS, Kim HM, Hong YS, Yoon SA, Kang SW, Kim JH, Kim MH, Ko HC, Kim SJ. Immature Citrus sunki peel extract exhibits antiobesity effects by beta-oxidation and lipolysis in high-fat diet-induced obese mice. Biol Pharm Bull. 2012; 35(2):223–230.

15. Cui ZG, Kim BY, Kang SH, Lee YJ, Lee DH, Lee YK, Park DB. Fermented peel of Citrus sunki Hort ex Tanaka promotes ethanol metabolism and suppresses body fat accumulation. Food Sci Biotechnol. 2007; 16(2):311–314.
16. Lan A, Xu W, Zhang H, Hua X, Zheng D, Guo R, Shen N, Hu F, Feng J, Liu D. Inhibition of ROS-activated p38MAPK pathway is involved in the protective effect of H2S against chemical hypoxia-induced inflammation in PC12 cells. Neurochem Res. 2013; 38(7):1454–1466.

17. Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013; 87(7):1157–1180.

18. Ho SC, Kuo CT. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium). Food Chem Toxicol. 2014; 71:176–182.

19. Jung JY, Mo HC, Yang KH, Jeong YJ, Yoo HG, Choi NK, Oh WM, Oh HK, Kim SH, Lee JH, Kim HJ, Kim WJ. Inhibition by epigallocatechin gallate of CoCl2-induced apoptosis in rat PC12 cells. Life Sci. 2007; 80(15):1355–1363.

20. Jung JY, Roh KH, Jeong YJ, Kim SH, Lee EJ, Kim MS, Oh WM, Oh HK, Kim WJ. Estradiol protects PC12 cells against CoCl2-induced apoptosis. Brain Res Bull. 2008; 76(6):579–585.

21. Zeng KW, Wang XM, Ko H, Yang HO. Neuroprotective effect of modified Wu-Zi-Yan-Zong granule, a traditional Chinese herbal medicine, on CoCl2-induced PC12 cells. J Ethnopharmacol. 2010; 130(1):13–18.

22. Cervellati F, Cervellati C, Romani A, Cremonini E, Sticozzi C, Belmonte G, Pessina F, Valacchi G. Hypoxia induces cell damage via oxidative stress in retinal epithelial cells. Free Radic Res. 2014; 48(3):303–312.

23. Wang LX, Zeng JP, Wei XB, Wang FW, Liu ZP, Zhang XM. Effects of scutellarin on apoptosis induced by cobalt chloride in PC12 cells. Chin J Physiol. 2007; 50(6):301–307.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download