Abstract
BACKGROUND/OBJECTIVE
Chronic hyperglycemia induces oxidative stress via accumulation of reactive oxygen species (ROS) and contributes to diabetic complications. Hyperglycemia induces mitochondrial superoxide anion production through the increased activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. This study aimed to determine whether fisetin and luteolin treatments suppress the oxidative stress by modulating the expression of sirtuins (SIRTs) and forkhead box O3a (FOXO3a) under hyperglycemic conditions in human monocytes.
MATERIALS/METHODS
Human monocytic cells (THP-1) were cultured under osmotic control (14.5 mmol/L mannitol), normoglycemic (NG, 5.5 mmol/L glucose), or hyperglycemic (HG, 20 mmol/L glucose) conditions, in the absence or presence of fisetin and luteolin for 48 h. To determine the effect of fisetin and luteolin treatments on high glucose-induced oxidative stress, western blotting and intracellular staining were performed.
RESULTS
Hyperglycemic conditions increased the ROS production, as compared to normoglycemic condition. However, fisetin and luteolin treatments inhibited ROS production under hyperglycemia. To obtain further insight into ROS production in hyperglycemic conditions, evaluation of p47phox expression revealed that fisetin and luteolin treatments inhibited p47phox expression under hyperglycemic conditions. Conversely, the expression levels of SIRT1, SIRT3, SIRT6, and FOXO3a were decreased under high glucose conditions compared to normal glucose conditions, but exposure to fisetin and luteolin induced the expression of SIRT1, SIRT3, SIRT6, and FOXO3a. The above findings suggest that fisetin and luteolin inhibited high glucose-induced ROS production in monocytes through the activation of SIRTs and FOXO3a.
Hyperglycemia contributes to diabetes and several diabetic complications, such as retinopathy, neuropathy, and nephropathy [12]. High glucose (HG) has been shown to induce inflammatory cytokines, chemokines, p38 mitogen-activator protein kinase, reactive oxygen species (ROS), protein kinase C (PKC), and nuclear factor-κB (NF-κB) activity in both clinical and experimental systems [34]. Hyperglycemia increases the intracellular ROS formation, which is the prevailing mechanism leading to endothelial cell dysfunction, cellular damage and diabetic complications [5]. The phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase plays an important role in ROS production and consists of several proteins in resting cells, such as p47phox (NADPH oxidase cytosolic proteins), p67phox, and p40phox [6]. In our previous studies, we observed that hyperglycemia increased the p47phox expression and ROS generation [78].
Sirtuins are a class of proteins known as NAD-dependent deacetylases. The sirtuin family is divided into seven homologs (i.e., SIRT1-SIRT7) in mammals. Sirtuin affects a wide range of cellular processes, such as aging, apoptosis, inflammatory response, calorie restriction, energy efficiency, and stress resistance [9]. Previously, we reported that SIRT1 expression and superoxide anion production are altered in type 1 diabetes patients compared to normal healthy controls [8]. SIRT1 is a key mediator of various biological functions in mammals, including glucose-lipid metabolism, mitochondrial biogenesis, and inflammation [10].
The forkhead box O (FOXO) proteins are a family of transcription factors characterized by a conserved DNA-binding domain [11]. The members of the FOXO family, FOXO1 (FKHR), FOXO3 (FKHRL1), FOXO4 (AFX), and FOXO6, play important roles in apoptosis modulation, energy metabolism, DNA damage repair, cell differentiation, oxidative stress, and other cellular functions [12]. In addition, FOXO is the known substrate of SIRT1, which regulates the FOXO transcription factors through direct binding or deacetylation or both [1314]. However, there is little evidence on the exact interaction between SIRTs and FOXO under hyperglycemic conditions.
Flavonoids are reported to exert various biological activities such as anti-cancer, anti-inflammatory, and cardiovascular preventive effects [1516]. Several flavonoids have demonstrated anti-diabetic effects, including reduction of aldose reductase activity, regeneration of pancreatic cells, and enhancement of insulin release [17].
Fisetin (3,3′,4′,7-tetrahydroxyflavone), a bioactive flavonol found abundantly in fruits and vegetables, has chemopreventive, neuroprotective, anti-oxidant, anti-inflammatory, and other beneficial health effects [1819]. It also inhibits the expression of cell adhesion molecules, formation of ROS, and activation of NF-κB in vascular inflammatory responses [20]. Luteolin (3′,4′,5,7-tetrahydroxylflavone), a natural flavonoid found in fruits, vegetables, and medicinal herbs, also exerts anti-inflammatory, anti-allergic, anti-cancer, anti-oxidant, and other beneficial health effects [2122]. Luteolin is also known to improve blood glucose, HbA1c, insulin levels, and fatty acid metabolism-related gene expression [23]. Our previous studies revealed that under hyperglycemic conditions, both fisetin and luteolin (3-10 µM) inhibit pro-inflammatory cytokine production in monocytes via epigenetic changes involving NF-κB [2425]. However, the molecular mechanism of SIRTs and FOXO in regulating monocytic superoxide under hyperglycemic conditions in the presence and absence of dietary agents remains unknown. In this study, we hypothesize that under hyperglycemic conditions, fisetin and luteolin suppresses the ROS production through a mechanism involving modulation of p47phox and SIRTs/FOXO in human monocytes. This is the first report on the interactions between SIRTs and FOXO under diabetic conditions and exposure to fisetin and luteolin.
Fisetin and luteolin were purchased from Sigma Aldrich (St. Louis, MO, USA). Fisetin and luteolin stock solutions were prepared and stored in dimethyl sulfoxide (DMSO), and diluted with culture medium as required. The vehicle control in all experiments was 0.1% (v/v) DMSO. Anti-FoxO family antibodies were procured from Cell Signaling Technology (Beverly, MA, USA) and anti-SIRT1, SIRT3 and SIRT6 were procured from Abcam (Cambridge, MA, USA). The BCM™ protein assay kit was purchased from Pierce (Rockford, IL, USA). Novex pre-cast Tris-Glycine gels were obtained from Invitrogen (Carlsbad, CA, USA). All other chemicals, unless otherwise stated, were obtained from Sigma (St. Louis, MO, USA).
The human monocytic THP-1 cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). THP-1 cell were cultured in RPMI medium containing 10% fetal bovine serum and 1% antibiotics, and incubated at 37℃ in a 5% CO2 atmosphere. Fisetin and luteolin dissolved in DMSO were used for treatment of the cells. The final concentration of DMSO was 0.1% (v/v) for each concentration. THP-1 cells (1 × 105 cells/mL) were cultured in the presence of osmolar control (14.5 mM/L mannitol), normal glycemic (NG, 5.5 mM/L glucose) or hyperglycemic (HG, 20 mM/L glucose) conditions, in the absence or presence of fisetin (3, 5 and 10 µM) and luteolin (3, 5 and 10 µM) for 48 h. After 48h incubation, the medium was collected for measuring the cytokine release; the cell pellet was washed with phosphate-buffered saline (PBS) and cells were harvested for further experiments.
The toxicity of fisetin and luteolin on the cultured THP-1 cells was evaluated using the Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Rockville, MD, USA), according to the manufacturer's protocol. Briefly, THP-1 cells were seeded at 4 × 103 cells/well in a 96-well plate, and subsequently treated with various concentrations of the phytochemicals (3, 5 and 10 µM) for 48 h. Absorbance was measured using a Wallac EnVision microplate reader (PerkinElmer, Turku, Finland). The inhibitory effect of phytochemicals on the growth of cells was assessed as the percentage of cell growth reduction compared with vehicle-treated cells, which were defined as 100% viable.
After treatment with fisetin and luteolin, the medium was aspirated and cells were washed twice in PBS (10 mM, pH 7.4). Nuclear lysates were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL, USA). Lysates were collected and cleared by centrifugation, and the supernatant was aliquoted and stored at -80℃.
For western blot analysis, treated cells were homogenized at 4℃ in buffer containing 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% (v/v) Tween 20, 1 mM PMSF, and one protease inhibitor cocktail tablet (Roche, Basel-Stadt, Basel, Switzerland), and then centrifuged at 10,000 × g for 15 min. Protein concentration of the lysate was determined using the BCM™ protein assay kit. Samples (40 µg of total protein) were mixed with sample buffer (100 mM Tris-HCl, 2% sodium dodecyl sulfate, 1% 2-mercaptoethanol, 2% glycerol, and 0.01% bromophenol blue (pH 7.6)), incubated at 95℃ for 15 min, and loaded on 12% polyacrylamide gels. Electrophoresis was performed using the Mini Protean 3 Cell system (Bio-Rad, CA, USA). The resolved proteins were transferred on a nitrocellulose membrane (Schleicher & Schuell BioScience, Saxony, Dassel, Germany). For immunoblotting, the membranes were washed and incubated in blocking buffer (10 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% Tween 20, and 3% nonfat dry milk), and then incubated with diluted primary antibodies (1:1000) for 2 h at room temperature. Following incubation with the primary antibody, membranes were washed 3 times and then probed with diluted secondary antibodies (1:2000) for 1 h. The membranes were washed 3 times (15 min each) and detected by chemiluminescence ECL kit (Amersham Life Sciences Inc., Pittsburg, PA, USA).
Cells were cultured in a 6-well plate and incubated at 37℃ overnight to allow attachment, after which they were cultured overnight in serum-free medium. After treatment with fisetin and luteolin for 48h, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) (5 µM) was added and incubated with the cells for 15 min. The cells were then washed with 1 × PBS buffer, and fixed with 10% formaldehyde. Fluorescent images were captured with a fluorescent microscope. The signal quantification was assessed by ImageJ software.
Cytotoxic effects of fisetin and luteolin on THP-1 cells exposed to hyperglycemic conditions were investigated using the CCK-8 assay (Fig. 1). No toxicity was observed for both compounds at concentrations 3, 5 and 10 µM, at 48 h treatment under hyperglycemic conditions. All further experiments were performed in the non-toxic concentration range of fisetin and luteolin (3, 5 and 10 µM).
We examined the production of superoxide ions using immunofluorescence analysis (Fig. 2). Under hyperglycemic conditions, superoxide production was increased compared to normal glucose conditions. However, in the presence of fisetin and luteolin, the superoxide production decreased to levels similar to that under normal glucose conditions.
p47phox is a component of monocyte NADPH oxidase that produces ROS [26]. To obtain further insight into ROS production under hyperglycemic conditions, we examined whether fisetin and luteolin treatments suppressed the expression of the p47phox gene (Fig. 3). As expected, the expression of p47phox gene was greater in HG conditions than in NG conditions. The increased expression of p47phox gene was downregulated by luteolin (Fig. 3A) and fisetin (Fig. 3B) treatments.
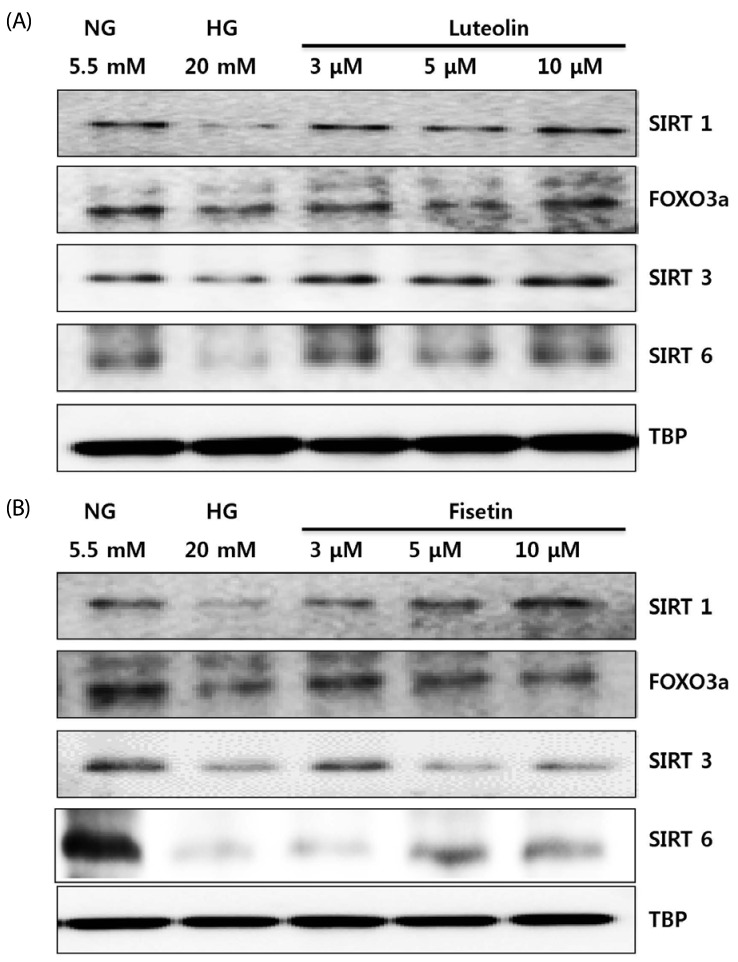
To identify the specific underlying mechanisms, we determined the effects of luteolin and fisetin on SIRT and FOXO3a expression in monocytes under hyperglycemic conditions. As shown in Fig. 4, SIRT1, SIRT3, SIRT6, and FOXO3a expression decreased significantly under hyperglycemic conditions as compared to normal glucose conditions. Conversely, SIRT1, SIRT3, SIRT6, and FOXO3a expressions were increased by luteolin (Fig. 4A) and fisetin (Fig. 4B) treatments.
Oxidative stress plays a crucial role in the development of diabetic complications such as atherosclerosis, cardiovascular disease, and coronary heart disease [5]. Diabetic patients show increased O2- production in monocytes. Hyperglycemia-induced excessive ROS accumulation contributes to diabetic complications [4567]. NADPH oxidase is accepted as the most important mechanism for ROS generation in phagocytic cells. Histone deacetylases (HDACs) are considered the key regulators of glucose metabolism, inflammation, and cell defense under various stress conditions. Sirtuin belongs to the class III HDACs (SIRT1-SIRT7) [9]. SIRT1 plays important roles in the regulation of glucose homeostasis by promoting insulin secretion and improving insulin resistance, protecting the pancreatic β-cells, controlling fatty acid oxidation and mitochondrial biogenesis, as well as decreasing inflammation, lipid mobilization, adiponectin excretion, and oxidative stress [891011]. Many studies suggest that SIRT1 is an effective therapeutic target for diabetes, and various natural compounds that can serve as activators of SIRT1 have been researched [10]. Loss of SIRT1 activity may be associated with metabolic diseases, such as diabetes [27]. Of the seven members of the sirtuin family, SIRT1, SIRT3, and SIRT6 play important roles in diabetes, inflammation, and oxidative stress [272829]. SIRT3 is localized to the mitochondria and regulates metabolism, adenosine triphosphate (ATP) generation, and multiple additional metabolic processes [28]. SIRT6 is a chromatin-associated deacetylase known to be involved in aging, inflammation, metabolism, and stress response [29]. Among the FOXO family, FOXO3a is closely related to oxidative stress, and upregulates the transcription of ROS scavenging enzymes superoxide dismutase 2 (SOD2, also known as MnSOD) and catalase [1112]. SIRT1 is especially associated with FOXO transcription factors and regulates gene-specific transcription [1314]. SIRT3 also enhance and deacetylase FOXO3a in mitochondria to prevent oxidative stress through DNA-binding to the MnSOD promoter [30]. Previously, we have shown that p47phox expression is increased in HG conditions [8]. In the present study, we observed for the first time that the excessive production of superoxide in HG conditions is downregulated by fisetin and luteolin treatments. In addition, we observed that the increased expression of p47phox under hyperglycemic conditions decreases by fisetin and luteolin treatments. Venogopal et al. [7] showed that p47phox, an essential component of monocyte NADPH oxidase, is required for ROS generation under HG conditions. In type 2 diabetes patients, there was significant decrease in the monocytic SIRT1 expression and increase in the p47phox expression, compared to the control [7]. Moreover, SIRT3 plays an important role in mitochondrial oxidative stress by reducing the ROS production. We have previously reported the role of SIRT1and FOXO3a in regulating monocytic superoxide under HG conditions [8]. However, the roles of SIRT3 and SIRT6 were not clearly understood in our previous study. In this study, we observed that SIRT1, SIRT3, SIRT6, and FOXO3a expression was decreased in hyperglycemia-induced monocytes. Conversely, the downregulated expressions of SIRT1, SIRT3, SIRT6, and FOXO3a were restored by fisetin and luteolin treatments to levels similar to normoglycemic conditions. Based on these findings, we suggest that hyperglycemia downregulates the SIRTs-FOXO3a pathway, resulting in p47phox expression and ROS generation. Fisetin and luteolin treatments under HG conditions modulate the SIRT1, SIRT3, SIRT6, and FOXO3a expressions, and subsequently decrease the p47phox expression and ROS production.
In conclusion, fisetin and luteolin inhibit ROS production through the activation of SIRT and FOXO3a expression. Therefore, we propose that fisetin and luteolin are potential agents for the prevention and treatment of diabetes and its associated complications.
References
1. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013; 93:137–188. PMID: 23303908.

2. Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005; 28:164–176. PMID: 15616252.

3. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414:813–820. PMID: 11742414.

4. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005; 54:1615–1625. PMID: 15919781.
5. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010; 107:1058–1070. PMID: 21030723.

6. El-Benna J, Dang PM, Gougerot-Pocidalo MA, Elbim C. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch Immunol Ther Exp (Warsz). 2005; 53:199–206. PMID: 15995580.
7. Venugopal SK, Devaraj S, Yang T, Jialal I. Alpha-tocopherol decreases superoxide anion release in human monocytes under hyperglycemic conditions via inhibition of protein kinase C-alpha. Diabetes. 2002; 51:3049–3054. PMID: 12351446.
8. Yun JM, Chien A, Jialal I, Devaraj S. Resveratrol up-regulates SIRT1 and inhibits cellular oxidative stress in the diabetic milieu: mechanistic insights. J Nutr Biochem. 2012; 23:699–705. PMID: 21813271.

9. Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012; 13:225–238. PMID: 22395773.

10. Kitada M, Koya D. SIRT1 in type 2 diabetes: mechanisms and therapeutic potential. Diabetes Metab J. 2013; 37:315–325. PMID: 24199159.

11. Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013; 14:83–97. PMID: 23325358.

12. Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008; 27:2320–2336. PMID: 18391974.

13. Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med. 2005; 16:237–243. PMID: 16012755.

14. Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004; 303:2011–2015. PMID: 14976264.

15. Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm Allergy Drug Targets. 2009; 8:229–235. PMID: 19601883.

16. Peterson JJ, Dwyer JT, Jacques PF, McCullough ML. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr Rev. 2012; 70:491–508. PMID: 22946850.

17. Nicolle E, Souard F, Faure P, Boumendjel A. Flavonoids as promising lead compounds in type 2 diabetes mellitus: molecules of interest and structure-activity relationship. Curr Med Chem. 2011; 18:2661–2672. PMID: 21568900.

18. Li J, Cheng Y, Qu W, Sun Y, Wang Z, Wang H, Tian B. Fisetin, a dietary flavonoid, induces cell cycle arrest and apoptosis through activation of p53 and inhibition of NF-kappa B pathways in bladder cancer cells. Basic Clin Pharmacol Toxicol. 2011; 108:84–93. PMID: 21054790.

19. Currais A, Prior M, Dargusch R, Armando A, Ehren J, Schubert D, Quehenberger O, Maher P. Modulation of p25 and inflammatory pathways by fisetin maintains cognitive function in Alzheimer's disease transgenic mice. Aging Cell. 2014; 13:379–390. PMID: 24341874.
20. Kwak S, Ku SK, Bae JS. Fisetin inhibits high-glucose-induced vascular inflammation in vitro and in vivo. Inflamm Res. 2014; 63:779–787. PMID: 24923846.

21. Xia F, Wang C, Jin Y, Liu Q, Meng Q, Liu K, Sun H. Luteolin protects HUVECs from THF-α-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-κB and MAPK pathways. J Atheroscler Thromb. 2014; 21:768–783. PMID: 24621786.
22. Seelinger G, Merfort I, Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008; 74:1667–1677. PMID: 18937165.

23. Zang Y, Igarashi K, Li Y. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-A(y) mice. Biosci Biotechnol Biochem. 2016; 80:1580–1586. PMID: 27170065.
24. Kim HJ, Kim SH, Yun JM. Fisetin inhibits hyperglycemia-induced proinflammatory cytokine production by epigenetic mechanisms. Evid Based Complement Alternat Med. 2012; 2012:639469. PMID: 23320034.

25. Kim HJ, Lee W, Yun JM. Luteolin inhibits hyperglycemia-induced proinflammatory cytokine production and its epigenetic mechanism in human monocytes. Phytother Res. 2014; 28:1383–1391. PMID: 24623679.

26. El-Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009; 41:217–225. PMID: 19372727.

27. Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol. 2009; 5:367–373. PMID: 19455179.

28. Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010; 143:802–812. PMID: 21094524.

29. Liao CY, Kennedy BK. SIRT6, oxidative stress, and aging. Cell Res. 2016; 26:143–144. PMID: 26780861.

30. Tseng AH, Shieh SS, Wang DL. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med. 2013; 63:222–234. PMID: 23665396.

Fig. 1
Cytotoxicity of fisetin and luteolin under HG conditions.
Effect of fisetin and luteolin on cell viability after 48 h was evaluated by the CCK-8 assay. Human monocyte THP-1 cells (4 × 103 cells/well) were cultured under normoglycemic (NG, 5.5 mM/L glucose) or hyperglycemic (HG, 20mM/L glucose) conditions in a 96-well plate. Cells were treated with 3, 5 and 10 µM luteolin and fisetin for 48 h. Results are shown as mean ± SE of three independent experiments.
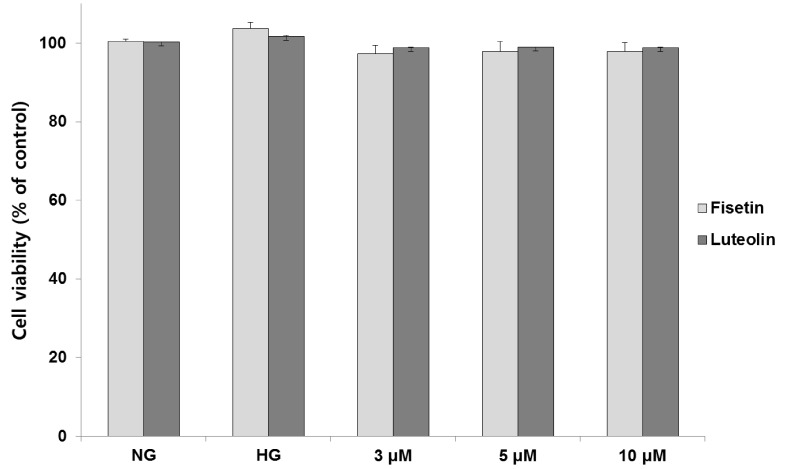
Fig. 2
Effects of luteolin and fisetin on O2- production in HG conditions by immunofluorescence.
Cells were treated with appropriate concentrations of the phytochemicals, and fixed with 10% formaldehyde. The signal quantification was assessed by ImageJ software; magnification ×400. Man, mannitol condition; NG, normoglycemic condition; HG, hyperglycemic condition; L, luteoline; F, fisetin.
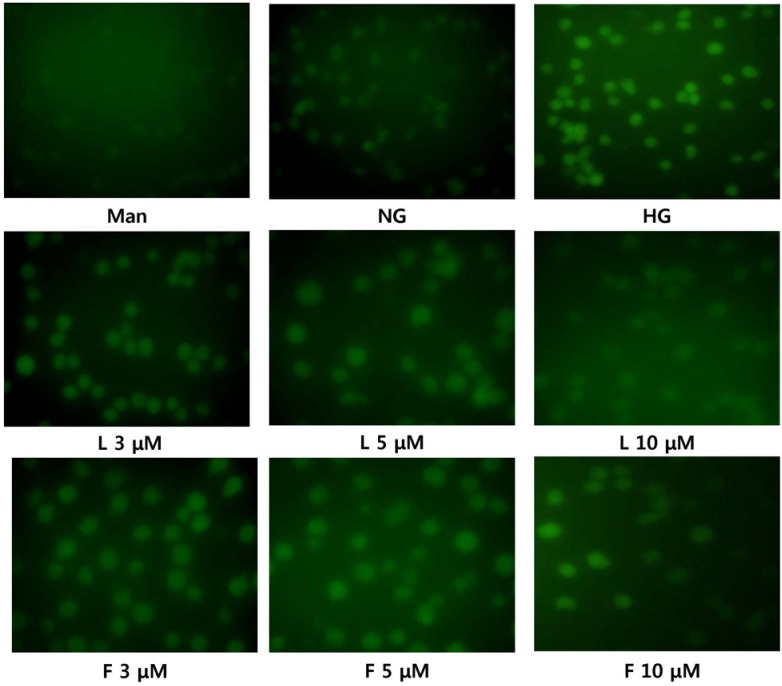
Fig. 3
Effects of luteolin and fisetin on p47phox expression in HG conditions.
Cells were treated with 3, 5 and 10 µM luteolin (A) and fisetin (B) for 48 h. Protein levels were evaluated by western blot for p47phox. Equal loading of protein was confirmed by stripping the immunoblot and reprobing for β-actin protein. The immunoblots shown here are representative of three independent experiments. NG, normoglycemic condition; HG, hyperglycemic condition.
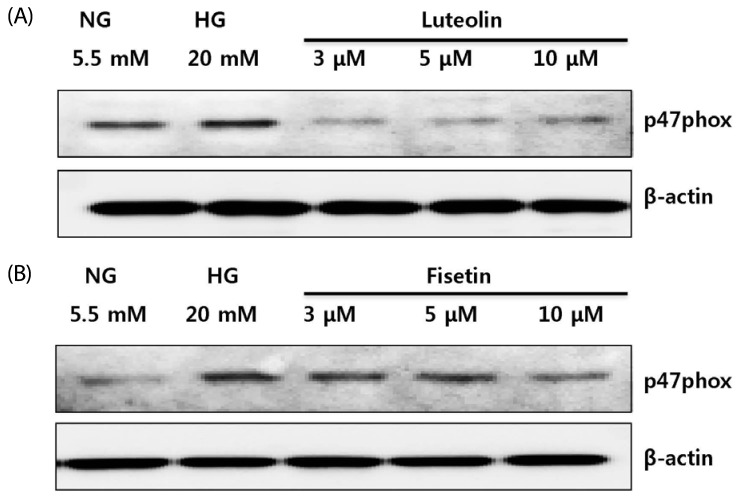
Fig. 4
Effects of luteolin and fisetin on SIRTs and FOXO3a gene expression under HG conditions
Cells were treated with 3, 5 and 10 µM luteolin (A) and fisetin (B) for 48 h. Protein levels were evaluated by western blot for SIRT1, SIRT3, SIRT6 and FOXO3a. Equal loading of protein was confirmed by stripping the immunoblot and reprobing for TATA binding protein (TBP). The immunoblots shown here are representative of three independent experiments. NG, normoglycemic condition; HG, hyperglycemic condition.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download