Abstract
Luteolin is a flavonoid found in abundance in celery, green pepper, and dandelions. Previous studies have shown that luteolin is an anti-inflammatory and anti-oxidative agent. In this study, the anti-inflammatory capacity of luteolin and one of its glycosidic forms, luteolin-7-O-glucoside, were compared and their molecular mechanisms of action were analyzed. In lipopolysaccharide (LPS)-activated RAW 264.7 cells, luteolin more potently inhibited the production of nitric oxide (NO) and prostaglandin E2 as well as the expression of their corresponding enzymes (inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) than luteolin-7-O-glucoside. The molecular mechanisms underlying these effects were investigated to determine whether the inflammatory response was related to the transcription factors, nuclear factor (NF)-κB and activator protein (AP)-1, or their upstream signaling molecules, mitogen-activated protein kinases (MAPKs) and phosphoinositide 3-kinase (PI3K). Luteolin attenuated the activation of both transcription factors, NF-κB and AP-1, while luteolin-7-O-glucoside only impeded NF-κB activation. However, both flavonoids inhibited Akt phosphorylation in a dose-dependent manner. Consequently, luteolin more potently ameliorated LPS-induced inflammation than luteolin-7-O-glucoside, which might be attributed to the differentially activated NF-κB/AP-1/PI3K-Akt pathway in RAW 264.7 cells.
Lipopolysaccharide (LPS) triggers excessive secretion of a variety of inflammatory mediators including nitric oxide (NO) and prostaglandin E2 (PGE2). Elevated production of NO and PGE2 induced by LPS is mediated through the induction of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX-2) expression, which is in turn regulated by the transcription factors nuclear factor (NF)-κB and activator protein (AP)-1. These ubiquitously expressed transcription factors reside in the cytoplasm as a heterodimer with p50 and p65. The transcription factors are activated and translocated to the nucleus in response to inflammatory stimulus, resulting in upregulation of inflammation-related genes such as iNOS and COX-2 [1-3]. Therefore, dietary phytochemicals that modulate iNOS and COX-2 expression by suppressing transcription factors may act as anti-inflammatory agents.
The flavonoid family is a ubiquitous form of polyphenol that is ingested by humans with a regular diet. More than 4,000 individual flavonoid compounds have been identified to date, and many studies have attempted to identify their pharmaceutical applications with regard to their anti-oxidative and anti-inflammatory effects [4-6]. Flavonoids in plants are usually present in the form of glycosides, although they are found as aglycones. It is well known that glycosides are hydrolyzed by the β-glucosidase enzyme produced by gut bacteria and subsequently absorbed as aglycones [7]. Aglycones are absorbed faster and in greater quantities than their glycoside counterparts in vitro [8, 9] and in vivo [10,11]. However, several contradictory findings have shown that glycosides can be absorbed without β-glucosidase hydrolysis and remain biologically active [12]. Interestingly, the bioavailability of flavonoids does not differ when consumed as either aglycones or glycosides [13,14]. Furthermore, after absorption flavonoid aglycones are reconjugated to glucuronic acid or sulfuric acid and metabolized [15,16], which should be considered when evaluating their efficacy in vitro. Nevertheless, numerous studies have focused on the biological activity of flavonoid aglycones [17,18]. Accordingly, it is necessary to compare the anti-inflammatory activity of aglycone and glycoside forms of flavonoids.
Luteolin (3',4',5,7-hydroxyl-flavone), which is the most abundant flavonoid in dandelions, has been reported to possess strong anti-oxidative and anti-inflammatory activities [15,17,19]. Our previous studies confirmed that extracts from the aerial part of dandelion inhibit LPS-stimulated inflammation processes in RAW 264.7 cells and that luteolin is a major anti-inflammatory component [20,21]. In addition to free luteolin, luteolin-7-O-glucoside and luteolin-7-O-rutinoside were identified in the leaves and flowers of dandelion [22]. However, the biological activities of luteolin and luteolin glycosides have not been compared. Therefore, in this study, the anti-inflammatory activities of luteolin and luteolin-7-O-glucoside, the aglycone and glucoside form of luteolin, were compared and their underlying molecular mechanisms in LPS-stimulated RAW 264.7 cells were investigated.
Dulbecco's modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco BRL (Gaithersburg, MD, USA). Luteolin, LPS, DMSO, and SDS were purchased from the Sigma Chemical Co. (St. Louis, MO, USA). Luteolin-7-O-glucoside was obtained from Chromadex (Irvine, CA, USA). Antibodies against iNOS, COX-2, phospho-p65, p65, phospho-c-jun, c-jun, phospho-extracellular signal-regulated kinase (ERK), ERK, phosphor-c-Jun NH2-terminal kinase (JNK), JNK, phospho-p38, p38, phosphor-Akt, Akt, and β-actin as well as horseradish peroxidase (HRP)-conjugated anti-rabbit IgG were purchased from Cell Signaling (Boston, MA, USA). All other chemicals were of the highest commercial grade available.
The RAW 264.7 murine macrophage cell line was obtained from the American Type Culture Collection (TIB-71; Rockville, MD, USA) and cultured in DMEM supplemented with 10% FBS and 2 mM l-glutamine. Cells in 100 mm dishes (5 × 106 cells/dish) or 24 well plates (4 × 105 cells/well) were pre-incubated with and without the indicated concentrations of luteolin and luteolin-7-O-glucoside (5, 10, 25, 50 µM) for 2 h, then incubated with LPS (1 µg/ml) for 20 h at 37℃ in a humidified atmosphere containing 5% CO2.
Cell viability was quantified using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazoliu m inner salt (MTS) assay purchased from Promega (Madison, WI, USA). Briefly, cells were seeded onto a 24-well plate and then incubated for 18 h before treatment to allow them to attach to the bottom of the well. After agents were applied at the indicated concentrations, the cell viability was measured according to the manufacturer's instructions. Next, 50 µl of MTS solution were added to 950 µl of culture media and incubated for 1 h at 37℃, after which the optical density was measured at 490 nm.
The nitrite accumulated in the culture medium is an indicator of NO production and was measured according to the Griess reaction. Briefly, 100 µl of each medium supernatant was mixed with 50 µl sulphanilamide (1% in 5% phosphoric acid) and 50 µl naphthylenediamine dihydrochloride (0.1%) and then incubated at room temperature for 10 min. The absorbance at 550 nm was then measured against a NaNO2 serial dilution standard curve and nitrite production was determined. The PGE2 concentration in the cell culture supernatant was measured using an enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instructions.
Cells plated in 100-mm dishes were pre-incubated with and without the indicated concentrations of each sample for 2 h, after which they were incubated with LPS for 18 h. The cells were then washed twice with PBS, scraped into 0.4 ml of ice-cold protein extraction solution (Intron Biotechnology, Seongnam, Korea) and placed on ice for 10 min. Next, the disrupted cells in lysis buffer were centrifuged at 13,000 × g for 5 min. Protein samples (50 µg) from each lysate were subsequently separated on a 10% SDS-polyacrylamide gel and electro-transferred to a PVDF membrane (Bio-Rad, Hercules, CA, USA), after which membranes were blocked for 1 h at room temperature with 5% nonfat dry milk in TBST solution. The reactions were then incubated at 4℃ overnight with 1:1000 dilutions of each primary antibody. Following overnight incubation, the membranes were washed and then incubated with a 1:1000 dilution of horseradish peroxidase-conjugated anti-rabbit IgG for 2 h at room temperature. The blots were then developed with ECL developing solution (Santa Cruz Biotechnology, Santa Cruz, CA, USA), after which the data were quantified using the Gel Doc EQ System (Bio-Rad).
To identify sub-cellular localization of transcription factors, nuclear and cytoplasmic extracts were prepared using an NEPER™ nuclear and cytoplasmic extraction reagents kit (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer's instructions. Next, 25 µg of lysate from each preparation were separated by SDS-PAGE and then subjected to Western blot analysis as described above.
All data are expressed as the mean ± S.D. Statistical analyses were conducted using SPSS version 10.0 (SPSS Institute, Chicago, IL, USA). One-way ANOVA with Duncan's multiple-range test was used to examine the difference between groups. P values < 0.05 were considered significant unless otherwise stated.
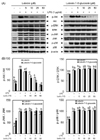
In this study, we compared the anti-inflammatory activity of luteolin and luteolin-7-O-glucoside in LPS-stimulated RAW 264.7 cells and investigated the mechanisms underlying this activity. Notably, both flavonoids inhibited LPS-induced NO (Fig. 1A) and PGE2 (Fig. 1B) production in a dose-dependent manner, without any cytotoxicity (Fig. 1C). Luteolin exhibited more potent suppression of NO and PGE2 than luteolin-7-O-glucoside (Fig. 1A and 1B). The IC50 values of NO and PGE2 in response to luteolin and luteolin-7-O-glucoside treatment were 13.9 µM and 22.7 µM for NO and 7.4 µM and 15.0 µM for PGE2, respectively. Western blot analysis was performed to analyze the levels of iNOS and COX-2 protein expressions, which are involved in NO and PGE2 production, respectively. Both flavonoids inhibited LPS-induced expression of iNOS and COX-2 in a dose-dependent manner (Fig. 2A and 2B). Stronger suppression of the expression of these proteins was observed in luteolin-treated macrophages than in luteolin-7-O-glucoside-treated macrophages.
NF-κB and AP-1 are important transcription factors that control the expression of inflammatory mediators such as iNOS and COX-2. An inflammatory stimulus induces the phosphorylation of p65 and c-jun, which then release NF-κB and AP-1, while p65 and c-jun are trans-located into the nucleus [4]. To investigate whether the anti-inflammatory effects of luteolin and luteolin-7-O-glucoside occur through the NF-κB and AP-1 pathways, p65 and c-jun phosphorylation and their nuclear translocation were measured by Western blotting. As shown in Fig. 3A and 3B, phosphorylation of both p65 and c-jun was suppressed by luteolin treatment, and only p65 was phosphorylated with luteolin-7-O-glucoside treatment. Therefore, nuclear translocation of p65 and c-jun were suppressed by luteolin, resulting in more p65 and c-jun in the cytoplasmic fraction than in the nuclear fraction (Fig. 4A and 4B). However, translocation of p65 into the nucleus was inhibited by luteolin-7-O-glucoside, while translocation of c-jun into the nucleus was slightly, but not significantly, decreased by luteolin-7-O-glucoside treatment (Fig. 4C and 4D).
Phosphorylation of MAPKs (ERK, JNK, p38, and Akt) was measured by Western blotting to identify the upstream regulatory mechanisms underlying NF-κB and AP-1 inactivation. As shown in Fig. 5A and 5B, luteolin and luteolin-7-O-glucoside inhibited Akt phosphorylation, but did not affect ERK, JNK, or p38 phosphorylation. These findings suggest that inhibition of Akt phosphorylation by luteolin and luteolin-7-O-glucoside treatment results in decreased expression of LPS-induced inflammatory transcription factors and mediators in RAW 264.7 cells (Fig. 6).
Luteolin and luteolin-7-O-glucoside-treated LPS-stimulated RAW 264.7 cells exhibit different anti-inflammatory capacities and molecular mechanisms. Inflammation is the tissue's response to injury and can be caused by inflammatory cytokines or chemokines, generation of reactive oxygen species, and secretion of LPS. Over-production of NO via iNOS and PGE2 by COX-2 are common markers of inflammation [4]. In this study, luteolin and luteolin-7-O-glucoside inhibited NO and PGE2 production in a dose-dependent manner. Luteolin more strongly inhibited NO and PGE2 production, as well as expression of their corresponding enzymes, iNOS and COX-2, than luteolin-7-O-glucoside. The IC50 values of luteolin and luteolin-7-O-glucoside were 13.9 µM and 22.7 µM for NO and 7.4 µM and 15.0 µM for PGE2, respectively. These results suggest that the free form of luteolin exhibits stronger anti-inflammatory activity than luteolin glycoside in LPS-stimulated RAW 264.7 cells. Previous studies have also confirmed the anti-oxidative and anti-inflammatory activities of luteolin [15,17,18]. In addition, luteolin glucosides have been shown to possess anti-asthmatic [23], anti-UV [24], chemopreventive [25], and anti-inflammatory [26] activities. However, no studies comparing the biological efficacy of luteolin and luteolin-7-O-glucoside have been conducted to date. The results of the present study showed that luteolin-7-O-glucoside was less effective at suppressing NO and PGE2 production than free luteolin. This difference might have been due to variation in the cellular uptake of luteolin and luteolin-7-O-glucoside. Murota et al. [8] found that the aglycone form of isoflavone was taken up into Caco-2 cells more efficiently than the glucoside form because of its moderate lipophilicity. Several researchers have also confirmed that flavonoid glucosides are not easily absorbed because they are bound to sugars such as β-glucosides in vivo [10,11]. Most flavonoids present in plants are attached to sugars, although they are occasionally found as aglycones. Effective absorption of flavonoids likely requires the conversion of glucosides to aglycones by β-glycosidase in vivo. It seems reasonable that aglycones are absorbed faster than glucosides in vitro because they have greater hydrophobicity and a smaller molecular weight, whereas glucosides have lower absorbability since they must be converted to aglycones for absorption in vivo [11]. However, few human studies have shown similar or greater uptake efficiency of flavonoid glucosides relative to their corresponding aglycones [27,28]. Hollman and Katan [28] found that the human absorption of quercetin glucosides from onions (52%) is greater than that of the pure aglycones (24%) and suggested that the sugar moiety is an important determinant of absorption and bioavailability. Zhou et al. [9] reported that the permeability and absorption rate constant of luteolin from peanut hull extract were significantly greater than those of pure luteolin. Zubik and Meydani [14] also reported that the apparent bioavailability did not differ between aglycones or glucosides in humans. These findings imply that flavonoid glucosides in foods are easily hydrolyzed by β-glycosidase and absorbed as effectively as free flavonoids. Furthermore, absorbed aglycones are reconjugated to glucuronic acid, and to a lesser degree to sulfuric acid. Only a small portion of free aglycone has been detected in blood, demonstrating that the rate of conjugation is high [16]. This means that the aglycone is necessary for absorption through enterocytes, but that the conjugated form is prevalent after nutritional uptake [15]. Based on these reports, the discrepancy of bioavailability between in vitro and in vivo studies may be partly attributed to the existence of β-glycosidase in the small and large intestine. These findings also indicate that the biological activities of aglycones and glycosides in vitro might be different from those in vivo.
NF-κB and AP-1 are transcription factors that regulate the expression of genes involved in inflammation, differentiation, and proliferation. Activation of NF-κB and AP-1 is highly associated with the induction of inflammatory enzymes, including iNOS and COX-2. Therefore, NF-κB and AP-1 are considered critical targets for treatments that inhibit inflammatory responses [3, 4]. AP-1 is a ubiquitous protein that resides in the cytoplasm as homo- or heterodimers with the jun and fos families, while NF-κB is composed of p65 and p50. Activation of NF-κB and AP-1 is regulated by the inducible phosphorylation of p65 and c-jun, which are subunits of NF-κB and AP-1, respectively [1,4]. As shown in Fig. 3, luteolin suppressed p65 and c-jun phosphorylation, while luteolin-7-O-glucoside only suppressed p65 phosphorylation. In addition, nuclear translocation of both transcription factors coincided with their phosphorylated status. That is, luteolin suppressed iNOS and COX-2 expression through the inhibition of NF-κB and AP-1, while luteolin-7-O-glucoside acted through NF-κB, but not AP-1. Furthermore, suppression of NF-κB by luteolin was stronger than that induced by luteolin-7-O-glucoside. These findings might partially explain why luteolin-7-O-glucoside weakly mitigated LPS-induced iNOS and COX-2 expression relative to the strong repression obtained with luteolin treatment. While it is not known how luteolin and luteolin-7-O-glucoside behave through different mechanisms to modulate inflammation process, many researchers have studied the transcription factors involved in the anti-inflammatory activity of luteolin in various cell lines [15,29-31]. Several researchers have reported that luteolin inhibits NO production by inactivating NF-κB [15,19,21], AP-1 [30], or both [17,18,31]. There is also intriguing evidence that NF-κB and AP-1 modulate each other, thus expanding the scope of these two rapidly inducible transcription factors [2].
There has been considerable progress in understanding the signaling pathways involved in NF-κB and AP-1 regulation. NF-κB and AP-1 activity is regulated through interactions with specific protein kinases [4]. Previous studies have reported that LPS-induced NF-κB and AP-1 activation are regulated by a cascade of events that lead to the activation of MAPKs and Akt [20,21,29]. In this study, luteolin and luteolin-7-O-glucoside inhibited Akt phosphorylation, but did not affect ERK, JNK or p38 phosphorylation. Luteolin-mediated NF-κB and AP-1 suppression is the result of Akt inhibitory activity. Specifically, these results suggest that phospho-Akt inhibition by luteolin and luteolin-7-O-glucoside contribute to LPS-induced suppression of NF-κB and AP-1 activity, which results in reduced expression of inflammatory mediators in RAW 264.7 cells. Many researchers have investigated the effects of flavonoids on upstream signaling of inflammatory pathways in various cell lines [17,21,29]. Luteolin was reported to inhibit NO production by inactivating NF-κB and Akt in LPS-stimulated RAW 264.7 cells [17,21] and to reduce IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1 [30]. Interestingly, the protective effects of luteolin against LPS-induced acute lung injury involve inhibition of the MEK/ERK and PI3K/Akt pathways in neutrophils [29]. Park et al. [31] found that luteolin modulates iNOS expression by inactivating NF-κB and AP-1 via the JNK pathway in HepG2 cells. Moreover, Oh et al. [32] showed that lutein inhibits activation of the redox-sensitive AP-1 pathway by suppressing the activation of p38 and JNK in skin keratinocytes. Overall, these findings suggest that the upstream signaling pathways that modulate transcription factors might differ depending on the flavonoid and cell line used.
Taken together, our findings strongly suggest that luteolin significantly inhibits NO and PGE2 production through the NF-κB/AP-1/PI3K-Akt signaling cascades, while luteolin-7-O-glucoside, which has relatively weak efficacy, functions through the NF-κB/PI3K-Akt signaling pathway in LPS-stimulated macrophages. In addition, we found that either luteolin aglycone or its glucoside is potential candidates for the treatment of inflammation.
Figures and Tables
Fig. 1
Luteolin and luteolin-7-O-glucoside inhibited NO (A) and PGE2 (B) production in LPS-stimulated RAW 264.7 cells without cytotoxicity (C). Data represent the mean ± SD of triplicate experiments. Values sharing the same superscript are not significantly different at P < 0.05 by Duncan's multiple range test. Lu: Luteolin, Lu-7-G: luteolin-7-O-glucoside.
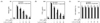
Fig. 2
Luteolin and luteolin-7-O-glucoside inhibited protein expression of iNOS and COX-2 in LPS-stimulated RAW 264.7 cells. Panel A shows protein expression levels of iNOS and COX-2 in response to luteolin and luteolin-7-O-glucoside. All signals were normalized to protein levels of β-actin, an internal control, and expressed as a ratio (Panel B). Data represent the mean ± SD of triplicate experiments. Values sharing the same superscript are not significantly different at P < 0.05 by Duncan's multiple range test. Lu: Luteolin, Lu-7-G: luteolin-7-O-glucoside.
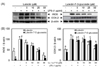
Fig. 3
Luteolin and luteolin-7-O-glucoside inhibited phosphorylation of transcription factors, NFκB and AP-1, in LPS-stimulated RAW 264.7 cells. Panel A shows phosphorylated status of p65 and c-jun in response to luteolin and luteolin-7-O-glucoside. All signals were normalized to protein levels of β-actin, an internal control, and expressed as a ratio (Panel B). Data represent the mean ± SD of triplicate experiments. Values sharing the same superscript are not significantly different at P < 0.05 by Duncan's multiple range test. Lu: Luteolin, Lu-7-G: luteolin-7-O-glucoside.
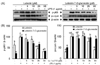
Fig. 4
Luteolin and luteolin-7-O-glucoside inhibited translocation of p65 and c-jun in LPS-stimulated RAW 264.7 cells. Panel A and C show translocation of p65 and c-jun in response to luteolin and luteolin-7-O-glucoside. All signals were normalized to protein levels of β-actin and PARP internal controls for cytosolic and nucleic extracts, and expressed as a ratio (Panel B and D). Data represent the mean ± SD of triplicate experiments. Values sharing the same superscript are not significantly different at P < 0.05 by Duncan's multiple range test. Lu-7-G: luteolin-7-O-glucoside.
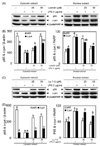
Fig. 5
Luteolin and luteolin-7-O-glucoside inhibited phosphorylation of Akt in LPS-stimulated RAW 264.7 cells. Panel A shows protein expression levels of p-Akt, p-ERK, p-JNK and p-p38 in response to luteolin and luteolin-7-O-glucoside. All signals were normalized to protein levels of Akt, ERK, JNK and p38 internal controls, an expressed as a ratio (Panel B). Data represent the mean ± SD of triplicate experiments. Values sharing the same superscript are not significantly different at P < 0.05 by Duncan's multiple range test.
References
1. Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001; 480-481:243–268.

2. Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol. 2004; 24:7806–7819.

3. Kim YW, West XZ, Byzova TV. Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med (Berl). 2013; 91:323–328.

5. Biesalski HK. Polyphenols and inflammation: basic interactions. Curr Opin Clin Nutr Metab Care. 2007; 10:724–728.

6. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000; 52:673–751.
7. Richelle M, Pridmore-Merten S, Bodenstab S, Enslen M, Offord EA. Hydrolysis of isoflavone glycosides to aglycones by β-glycosidase does not alter plasma and urine isoflavone pharmacokinetics in postmenopausal women. J Nutr. 2002; 132:2587–2592.

8. Murota K, Shimizu S, Miyamoto S, Izumi T, Obata A, Kikuchi M, Terao J. Unique uptake and transport of isoflavone aglycones by human intestinal Caco-2 cells: comparison of isoflavonoids and flavonoids. J Nutr. 2002; 132:1956–1961.

9. Zhou P, Li LP, Luo SQ, Jiang HD, Zeng S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J Agric Food Chem. 2008; 56:296–300.

10. Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000; 130:1695–1699.

11. Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006; 136:2291–2296.

12. Andlauer W, Kolb J, Fürst P. Isoflavones from tofu are absorbed and metabolized in the isolated rat small intestine. J Nutr. 2000; 130:3021–3027.

14. Zubik L, Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr. 2003; 77:1459–1465.

15. Seelinger G, Merfort I, Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008; 74:1667–1677.

16. Rowland I, Faughnan M, Hoey L, Wähälä K, Williamson G, Cassidy A. Bioavailability of phyto-oestrogens. Br J Nutr. 2003; 89:Suppl 1. S45–S58.

17. Chen CY, Peng WH, Tsai KD, Hsu SL. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007; 81:1602–1614.

18. Hu C, Kitts DD. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol Cell Biochem. 2004; 265:107–113.

19. Hwang JT, Park OJ, Lee YK, Sung MJ, Hur HJ, Kim MS, Ha JH, Kwon DY. Anti-tumor effect of luteolin is accompanied by AMP-activated protein kinase and nuclear factor-κB modulation in HepG2 hepatocarcinoma cells. Int J Mol Med. 2011; 28:25–31.

20. Park CM, Park JY, Noh KH, Shin JH, Song YS. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-κB modulation in RAW 264.7 cells. J Ethnopharmacol. 2011; 133:834–842.

21. Park CM, Jin KS, Lee YW, Song YS. Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-κB translocation in LPS stimulated RAW 264.7 cells. Eur J Pharmacol. 2011; 660:454–459.

22. Schütz K, Kammerer DR, Carle R, Schieber A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2005; 19:179–186.

23. Jin M, Yang JH, Lee E, Lu Y, Kwon S, Son KH, Son JK, Chang HW. Antiasthmatic activity of luteolin-7-O-glucoside from Ailanthus altissima through the downregulation of T helper 2 cytokine expression and inhibition of prostaglandin E2 production in an ovalbumin-induced asthma model. Biol Pharm Bull. 2009; 32:1500–1503.

24. Verschooten L, Smaers K, Van Kelst S, Proby C, Maes D, Declercq L, Agostinis P, Garmyn M. The flavonoid luteolin increases the resistance of normal, but not malignant keratinocytes, against UVB-induced apoptosis. J Invest Dermatol. 2010; 130:2277–2285.

25. Baskar AA, Ignacimuthu S, Michael GP, Al Numair KS. Cancer chemopreventive potential of luteolin-7-O-glucoside isolated from Ophiorrhiza mungos Linn. Nutr Cancer. 2011; 63:130–138.
26. Jung HA, Jin SE, Min BS, Kim BW, Choi JS. Anti-inflammatory activity of Korean thistle Cirsium maackii and its major flavonoid, luteolin 5-O-glucoside. Food Chem Toxicol. 2012; 50:2171–2179.

27. Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002; 22:19–34.

28. Hollman PC, Katan MB. Health effects and bioavailability of dietary flavonols. Free Radic Res. 1999; 31:Suppl. S75–S80.

29. Lee JP, Li YC, Chen HY, Lin RH, Huang SS, Chen HL, Kuan PC, Liao MF, Chen CJ, Kuan YH. Protective effects of luteolin against lipopolysaccharide-induced acute lung injury involves inhibition of MEK/ERK and PI3K/Akt pathways in neutrophils. Acta Pharmacol Sin. 2010; 31:831–838.

30. Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci U S A. 2008; 105:7534–7539.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download