Introduction
The phylum Acanthocephala includes at least 1,150 species of relatively small vermiform endoparasites. The adult members of the phylum locate and feed on the intestinal walls of fresh and marine water vertebrate fishes [1]. They are well adapted parasites with low specificities to their intermediate, definitive and transport hosts. A broader environmental tolerance of these parasites might account for the high degree of morphologic variability often found in species of this group [25]. Adult members are highly specialized heterosexual parasites that locate in the intestines and take up their nutrition through their teguments, since they have no intestine. These parasites are harmful to their host [6,20]. In general, many studies have been carried out on the fish parasites typical of Egypt, but few have focused on the acanthocephalans [2,8,11,12,15,23]. Most of these studies dealt with taxonomic features and little on the pathological changes visible by light microscopy. Recently, scanning electron microscopy has proved helpful in studying the tegument structures and prompted speculation on the possible functions of these structures [24]. The present study was conducted to obtain data on the surface topography of Acanthogyrus (Acanthosentis) tilapiae [7], to characterize morphologic features of the tegument, and to assess the pathological effects of this parasite based upon adaptation at the site of infection.
Materials and Methods
Acanthocephalan parasites were isolated and dissected from the intestines of 100 each of three tilapia fish species (Oreochromis niloticus, Sarotherodon gallileus and Tilapiae zilli). The fish were collected during the period November 2005 to April 2006, out of the River Nile at Giza governorate. The fish were anesthetized and in preparation for light microscopy, the intestinal parasites were identified and removed using binocular microscopy, the samples placed in tap water until their proboscis averted and then fixed in 10% formalin and washed repeatedly with distilled water to dilute and remove excess fixative. An acetic acid alum carmine preparation was used (10-30min) for staining [9]. Dehydration was performed using a step-wise series of increasing ethyl alcohol, cleared in clove oil and the specimens mounted in Canada balsam. The parasite measurements and mean were out of 10 specimens (± SD). The histopathology studies used tissue samples removed as sections of the intestine that harbored parasites and evidenced macroscopic lesions. The tissue samples were immersed in 10% formalin saline for 48 h and the sections for histopathology prepared, stained (hematoxylin & eosin) and mounted [9]. In preparation for scanning electron microscopy (SEM), live isolated parasite specimens were kept for 30 min in a refrigerator prior to their fixation in a 4% aqueous glutaraldehyde solution (4℃ for 48 h) [18]. The specimens were then washed thoroughly with cacodylate buffer and post-fixed for 4 h with aqueous osmium tetroxide, dehydrated through acetone, and dried in a critical point drying apparatus (Polaron E300; Polaron Equipment, UK) using liquid CO2. The specimens were whole-mounted on an aluminum stub and fixed by double-phase sticker. The specimens were then coated with gold-palladium in a sputter coating unit (Polaron E5000; Polaron Equipment, UK) and examined using a scanning electron microscope (JEOL SEM T330; JEOL, Japan) operating at 20 Kev.
Results
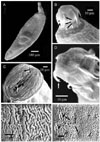
Acanthocephalan parasites were identified and isolated from the intestines of the three investigated fishes. The incidence of parasitic infestation of the fishes varied as follows: Oreochromis niloticus 78%, Sarotherodon gallileus 46%, Tilapiae zilli 24%. The parasite morphology assessments were based on the study of 15 whole mounted specimens. The parasites are described as club-shaped, elongated flukes, small to moderate in size with a total body length of 2.81 ± 0.03 mm and a maximum body width of 0.620 ± 0.030 mm. The body surface consisted of a series of folds and a large number of alternative pores (Fig. 1A&F). Grouped or scattered sensory ganglia were observed at the tegument and proboscis surfaces as shown in Fig. 1C. The proboscis is short, cylindrical and almost globular, measuring approximately 0.410 ± 0.020 mm in length and 0.390 ± 0.030 mm in diameter at the base (Fig. 2B&C) and characterized by three rings of curved hooks oriented towards the posterior, each ring possessing six hooks (Fig. 1D). The long hooks of the anterior ring are supported by simple unmodified hooks, all of a similar size, with a mean length of 0.025 ± 0.050 mm (Fig. 1B&D). The hooks of the second and third ring are smaller than of the anterior first one, and measure approximately 0.017 ± 0.003 mm in length. A narrow ganglion exists at the base of the proboscis followed by a point of attachment of two unequal blind and compact mononucleated leminisci, the longer and shorter measuring 0.813 ± 0.020 mm and 0.760 ± 0.020 mm in length, respectively (Fig. 2A). A reproductive system in the posterior half of the trunk extends to the posterior end of the bursa. Oblong testes of unequal size and usually slightly overlapping each other are contiguous with the cement gland. The anterior testis is 0.520 ± 0.030 mm long by 0.540 ± 0.050 mm wide, while the posterior testis measures 0.620 ± 0.040 mm long by 0.410 ± 0.030 mm wide (Fig. 2A). They are each followed by a cement gland measuring 0.320 ± 0.040 × 0.237 ± 0.020 mm length by width.
Histopathology analysis
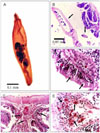
Histopathological changes of the infected intestinal fish indicated complete desquamation of the epithelium of the intestinal mucosa (Fig. 2C). Parasite attachment to the intestinal wall caused destruction of the villi, and degeneration and necrosis of the mucosal epithelium (Fig. 2B&C). Cell types resembling epitheloids, lymphocytes, macrophages and cells of unknown identity aggregated at the infected area in response to inflammation (Fig. 2C&E). The intestinal lumen contained cell debris and parasite structures (Fig. 2B). Severe hyperplasia of the goblet cells and a massive infiltration of leucocytic inflammatory cells were detected in focal manner between the lining mucosal epithelial cells (Fig. 2D&E). An inflammatory reaction at the submucosa of intestine, edema, mononuclear and eosinophilic focal infiltration were observed (Fig. 2E).
Discussion
The 8, 11, 26 and 37 species each of Acanthosentis have been documented by Petrochenko [22], Golvan [17], Yamaguti [26], Amin [3], respectively, and recently, Amin [4] added 2 more. At present, 44 species of Acanthosentis have been described, including the new species reported by Amin [4]. We have identified has the morphological features and measurements of Acanthogyrus (Acanthosentis) tilapiae; [7].
The present investigation, therefore, aimed to study the adaptative morphological characters of Acanthogyrus (Acanthosentis) tilapiae at the site of intestinal infection. The observations indicate that this Acanthocephalan has a cylindrical body with a multiple hooked proboscis through which attachment to the intestine of the host is maintained. The posterior oriented curved proboscis spines are considered the primary attachment organ as they are highly modified; whereas the larger hooks of the first row are supported by relatively unmodified hooks. The curvature and the presence of unmodified spines in the first row provide a good instrument for attachment. Also, the parasite appears to have sensory ganglia on the proboscis surface. It has been assumed, until recently, that penetration and destruction of intestinal cells were entirely mechanical due to the action of the proboscis spine [25]. Miller and Dunagan [21], however, described a pore like opening and groove on certain acanthocephalans and postulated delivery of a secretion via the spines. The present work documents that acanthocephalan penetration and destruction of the intestinal cells results from both a mechanical effect and a possible reaction to secretions. Regardless, highly modified spines for mechanical penetration and sensory ganglia to aid in releasing secretions for the destruction of intestinal cells could be possible. Due to the absence of a digestive system, the parasites absorb nutrients directly into the body [20]. Consequently, there are a great number of tegument folds and pores that increase the surface area and facilitate absorptive and excretory processes. The presence of sensory ganglia at the surface of the parasite act as sensory organs. The pathogenicity of acanthocephalans can be attributed to two factors: worm density and depth of worm penetration into host tissues [10]. The inflammatory response of the worm infested fish is dominated by granulocytes and macrophages, depending on the host species, and the structure of the proboscis hooks and tegument of the parasite [20]. The histopathology of the infested fish with Acanthogyrus (Acanthosentis) tilapiae, demonstrates the existence of inflammatory foci of mononuclear cells and intestinal hemorrhage. In addition, complete desquamation of the epithelium of the intestinal mucosa coupled with severe hyperplasia and hypertrophy of the goblet cells is commonly noted. It is speculated that the high inflammatory reaction in the submucosa, displacement of the sheaf associated with edema and cellular (mononuclear cells and eosinophils) infiltration reflect the host response. The hyperplastic goblet cells were disperse yet dominated the surface of villi, likely reducing the harmful local effects of the proboscis hooks and serving as an immune response against infection. Finally, the structure of proboscis hooks likely provoke the most obvious accumulation of the granulocytes i.e. the small rounded proboscis with large hooks attracting granulocytes in the acute initial phase of infestation in a focal manner [16,20]. Based on the accumulated observations, it is likely that the parasite we have identified with the proboscis features of curved and unmodified spines, a series of tegument folds and the presence of sensory ganglia in the proboscis and on the body surface define an organism suited to evoke a highly aggressive host reaction yet remain strongly anchored to its site of infestation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download