Abstract
A significant association between increased arterial stiffness and the development of cardiovascular disease has led to the increased use of arterial stiffness in the clinical assessment of cardiovascular risk. Various methods are currently available. With advances in technology, the assessment methods have become easy to use and more acceptable to patients. However, the different techniques that are available measure arterial stiffness at different locations and have unique indices for arterial stiffness. For the appropriate assessment of arterial stiffness, accurate and reproducible measurements of arterial stiffness are essential. Here we review the methodological aspects of the measurement of arterial stiffness and provide information on the measurement methods available and their clinical applications.
Blood pressure is largely influenced by cardiac contraction, large artery stiffness and the reflection of peripheral waves. During systole, cardiac contraction propels blood flow through the arterial system and this generates pressure waves that are propagated to all of the arteries in the body. When the pressure waves reach branch points, or sites of impedance mismatch, the pressure wave is reflected and returned to the heart. The reflected waves usually arrive during diastole at the heart and merge with the diastolic pressure wave.1)
When the arterial wall is compliant, the travel rate of the pressure pulse is relatively slow. Slow reflected pressure waves return to the central aorta during diastole, augmenting the diastolic blood pressure and the coronary blood flow. However, an increase in the arterial stiffness causes more rapid traveling pressure waves. In this situation, the reflected pressure waves return to the central aorta earlier and augment the central systolic blood pressure. Augmented central systolic blood pressure increases the left ventricular workload and compromises the coronary blood flow.2)
The importance of arterial stiffness on the development of cardiovascular disease has recently been appreciated. The measurement of arterial stiffness is frequently used to predict the risk of cardiovascular disease.3-5) Thus, the recently published guidelines for the management of hypertension have adopted measurement of arterial stiffness, by pulse wave velocity, for the evaluation of subclinical organ damage in hypertensive subjects.6) The clinical applications of arterial stiffness are as follows: 1) risk prediction of cardiovascular events; 2) endpoints in non-pharmacological and pharmacological studies; 3) evaluation of the hemodynamic changes observed in various clinical conditions and the pathogenesis of their cardiovascular complications. In the present paper, we review the methods used for the noninvasive assessment of arterial stiffness.
The contraction of the left ventricle ejects blood into the ascending aorta, dilating the aortic wall and generating a pressure pulse wave. The generated pressure pulse wave is propagated to the distal arterial tree at a different speed depending on the arterial segments. In stiffened arteries, the speed of the propagating pressure pulse wave becomes higher. The velocity of the pressure pulse wave propagation is based on the propagative model. The major determinants of the pulse wave velocity (PWV) are the elastic properties of the arterial walls and the geometry of the artery as well as the blood viscosity. Moens-Korteweg introduced an equation defining the PWV as follows: PWV2=(E·h)/(2r·ρ), where E is Young's modulus in the circumferential direction, h is the wall thickness, r is the radius, and ρ is the blood density.7) The PWV also can be calculated by measurement of the transit time of the pulse waves and the distance between two recording points. Therefore, the measurement of the arterial PWV is simple and reproducible.
The pulse waves in each artery (carotid, femoral, radial, and tibial arteries) can be recorded noninvasively with various sensors8-10) or continuous Doppler probes.11) The pulse wave transit time is the time delay between the proximal and distal pulse waves, as determined by the foot-to-foot method. The foot of the pulse wave is the point of minimal diastolic pressure or the systolic upstroke of the pulse wave. However, the precise determination of the foot of the pulse wave is difficult using a manual method. Currently, determination of the foot of the pulse way can be rapidly and easily performed with the aid of a computer. The "second derivative method" and the "intersecting tangent method" are the most reproducible computer algorithm available for the determination of the foot of the pulse wave.12) The distance between two recording points is measured over the body surface with a tape measure. The measured distance is not the true distance traveled by the pulse wave, but an estimate. The tape measurements may overestimate the distance in an obese person and underestimate the distance in patients with a tortuous aorta. The PWV is calculated with the measured pulse wave transit time (Δt) and distance (D) as follows (Fig. 1): PWV (cm/sec or m/sec)=Δt/D.
An average of 10 consecutive beats or the number of beats during a 10 second interval are evaluated for several respiratory cycles.8) The PWV can be measured at different locations: 1) the carotid-radial PWV, from carotid to radial artery; 2) the femoro-tibial PWV, from femoral to tibial artery; 3) the carotid-radial PWV, from carotid to femoral artery; 4) the brachial-ankle PWV, from brachial to tibial artery.
Recent advances in technology make measurement of the regional PWV easy. The Complior System (Artech Medical, Pantin, France) measures the PWV automatically with sensors applied directly onto the skin, obtaining pulse waves from two points simultaneously. This device is most widely used in epidemiology studies, and provides predictive information on the PWV for cardiovascular events. The SphygmoCor system (AtCor, Sydney, Australia) uses sequentially measured pulse waves from two sites, and the pulse wave transit time is calculated by gating to the peak of the R wave of the electrocardiogram.13) The pulse wave transit time is the subtraction of the time between the R waves and the proximal pulse wave from the time between the R waves and the distal pulse wave. A single high-fidelity applanation tonometer (Millar) is used to record the pulse waves. This device can also measure the central aortic blood pressure and augmentation index. The VP-1000/2000 (Colin Co, Komaki, Japan) is a unique device adopted for the measurement of the brachial-ankle PWV.10) Pulse waves from the brachial and tibial arteries are obtained with plethysmographic sensors incorporated into a blood pressure cuff that is wrapped around both of the arms and ankles.10) This device can also measure the central aortic PWV (heart-femoral PWV) and peripheral PWV (femoral-ankle PWV) with tonometric sensors.14)15) The results of small studies have shown that the brachialankle PWV was an independent predictor of cardiovascular death and cardiac events in elderly persons in the community16) as well as patients with coronary artery disease.17) Although data on the value of the brachial-ankle PWV for the prediction of cardiovascular events is limited, the brachial-ankle PWV is easy to measure and has the potential for screening applications.
The carotid-femoral PWV reflects the aortic PWV and is considered the 'gold-standard' measurement of arterial stiffness.18) The carotid-femoral PWV has been shown to be an independent predictor of cardiovascular morbidity and mortality in the general population,5) hypertensive patients,3) patients with end stage renal disease,4) and elderly subjects.19) In 2007, the European Society of Hypertension/European Society of Cardiology adopted the carotid-femoral PWV as a clinical variable for the stratification of cardiovascular risk in patients with hypertension.6) However, the interpretation of data from different methods of PWV measurement or meta-analyses should be considered with caution because the length that the measured pulse waves travel depends on the measurement methods. Moreover, there are many physiological factors affecting the PWV. The PWV depends on the blood pressure. An increase in blood pressure increases the PWV.8) An acute increase in the heart rate also elevates the PWV.20) These dynamic changes should be considered along with the changes in blood pressure when interpreting repeated measures of PWV in clinical trials or during the follow-up of patients.14) Age is also associated with an increase in the PWV; this association is independent of blood pressure and other cardiovascular risk factors.21)
Arterial distensibility and compliance are the expression of the relationship between the absolute or relative changes in the arterial volume and the distending pressure, given the assumption that the relationship between the changes in the luminal cross-sectional area and pressure are linear and that the length of the artery is constant during contraction.22) Arterial distensibility is expressed in terms of the relative change in the arterial volume for given pressure changes, and this is the inverse of the elastic modulus. However, in clinical practice, it is impractical to accurately measure the arterial volume and volume changes. Thus, for practical purpose, the arterial distensibility can be easily calculated as follows:
Distensibility coefficient (Pa-1 or mmHg-1)=ΔA/(ΔP×A)=(Dmax2-Dmin2)/D2×ΔP=(2ΔD×D+ΔD2)/D2×ΔP. Where ΔA is the difference in systolic and diastolic arterial cross-sectional area, ΔP is the pulse pressure, Dmax and Dmin are the maximum and minimum arterial diameter for the pressure changes, and ΔD is the difference in systolic and diastolic arterial diameter. D is either the end-diastolic or averaged arterial diameter.
Arterial compliance is the expression of the absolute change in the arterial volume for a given pressure change:
Compliance (cm2/mmHg or m2/Pa)=ΔA/ΔP=π (Dmax2-Dmin2)/4ΔP=π(2ΔD×D+ΔD2)/4ΔP. Incremental or Young's elastic modulus is another index of arterial stiffness and measures strain on the arterial wall.23) For calculation of the elastic modulus, changes in the arterial luminal diameter and arterial wall thickness are used and expressed as follows:
Incremental or Young's elastic modulus (mmHg/cm or Pa/cm)=(ΔP×D)/(ΔD×h) where h is the arterial wall thickness. Young's elastic modulus is the ratio of stress and strain on the arterial wall and a measure of intrinsic stiffness of the arterial wall.
Arterial stiffness is closely associated with blood pressure. The expression of arterial distensibility and compliance is a function of blood pressure. Some investigators have tried to calculate the arterial compliance independent of the blood pressure effect. This is referred to as the beta-stiffness index;24) there is a mathematical correction for the blood pressure effect.25) Because it is cumbersome, the mathematical correction is not commonly used. However, the beta stiffness index (β), which is relatively independent of transient blood pressure changes, using the logarithmic conversion of the ratio of systolic and diastolic blood pressure, is easier to use:
β=ln(SBP/DBP)×D/ΔD; where SBP is the systolic blood pressure and DBP is the diastolic blood pressure.
Ultrasound is commonly used to measure the arterial diameter. For this measurement, ultrasound uses an echo tracking method with radiofrequency tracking26) or Doppler processing27) for the detection of the displacement of the near and far walls of the arteries (Fig. 2). However, this method is too expensive and not practical for the clinical setting. A B-mode ultrasound can be used with automatic image processing.28) The systolic and diastolic diameter can also be measured manually from the B-mode image with electronic calipers and the aide of electrocardiography.29) Alternatively, the arterial elastic properties can be measured with the M-mode ultrasound.30) However, neither the B-mode or the M-mode ultrasound can measure the changes in the diameter accurately; however, they are not expensive and can be used in the clinical setting. The important issues to consider when using ultrasound for the evaluation of arterial stiffness include: operator dependent accuracy and the site of measurement of the blood pressure in relationship with the systemic application of locally determined arterial stiffness. A poor quality image cannot be used for accurate detection of changes in the arterial diameter. Another issue is that the blood pressure is needed for calculation of the arterial stiffness indices. Most studies use the brachial blood pressure when evaluating the aorta and carotid arterial stiffness; however, this assumes that the blood pressure of the aorta and carotid arteries is similar to the brachial artery. However, the pulse pressure is not constant along the arterial tree.31) The systolic pulse pressure of the peripheral muscular arteries is higher than the central elastic arteries, i.e. the aorta and carotid arteries. Therefore, investigators assess the carotid and aorta blood pressure with applanation tonometry using a transfer function. Whether locally measured arterial stiffness can reflect the stiffness of other arteries remains an unresolved issue. Arterial distensibility and compliance measures arterial stiffness with the assumption that the arterial segment is a cylindrical tube. However, the progression of atherosclerosis is not equally distributed among the arteries.32) Atherosclerosis of the common carotid artery is less common than at the carotid bifurcation and internal carotid artery.33)
In subjects with high blood pressure and/or diabetes, the carotid-femoral PWV and carotid stiffness does not provide similar information on the impact of aging on large artery stiffness.34) However, locally determined common carotid artery stiffness is moderately associated with aortic stiffness independent of the traditional cardiovascular risk factors.29) Clinical studies have shown that increased local arterial stiffness is significantly associated with increased cardiovascular risk in patients with end-stage renal disease;35) this suggests the potential utility of common carotid artery stiffness values for cardiovascular risk assessment.
The MRI can also be used for the measurement of aortic distensibility.36) Aortic distensibility in healthy subjects, evaluated by MRI, has been shown to be greatest in the ascending aorta, followed by the aortic arch and proximal descending aorta.36) However, MRI is expensive and not practical for routine clinic use.
Arterial compliance is defined as the relationship between the change in volume and the change in distending pressure. One of the methods used for measuring systemic arterial compliance is the "area method",37) expressed as follows:
Systemic arterial compliance=Ad/[R×(Ps-Pd)], where Ad is the area under the diastolic decay portion of the pulse pressure curve (from end systole to end diastole), R is the total peripheral resistance, Ps is the end-systolic aortic blood pressure, and Pd is the end-diastolic aortic blood pressure (Fig. 3). The pressure waveform representing the aortic root driving pressure is derived from the carotid artery waveform with applanation tonometry of the proximal right carotid artery. The central systolic blood pressure is derived from the calibration of the pressure obtained by tonometry against the brachial blood pressure measured simultaneously. The total peripheral resistance is calculated as the mean arterial blood pressure divided by the mean blood volume flow. The continuous flow velocity of the ascending aorta can be measured with the Doppler flow velocimeter placed on the suprasternal notch. The mean volume of flow is the product of the average systolic flow multiplied by the aortic root area measured by echocardiography. Another simpler method is the ratio of the stroke volume to the pulse pressure as follows:38)
Compliance=SV/ΔP, where SV is the stroke volume and ΔP is the pulse pressure. The stroke volume can be measured invasively or calculated with an equation. Although an accurate estimation of the stroke volume is difficult, the prognostic value of the stroke volume/pulse pressure has been reported.38) The capacitive (large artery) and oscillometric (small artery) systemic arterial compliance can be calculated with the use of pulse wave analysis and a modified Windkessel model (diastolic pulse contour analysis).39) A tonometric sensor is applied onto the radial artery and an oscillometric sensor onto the contralateral brachial artery. The validity of the modified Windkessel derived compliance is however doubted; this is because of the differences in the compliance of the arms and legs, which suggests a strong influence of regional circulatory properties.40) The methods used for the measurement of systemic arterial compliance are based on a theoretical model that is simplified for research purposes. The prognostic value of the systemic arterial compliance however has not been determined.
The arterial pressure wave is composed of a forward pressure wave that arises from the left ventricular ejection and a backward (reflected) pressure wave. The backward pressure occurs mainly as a consequence of wave reflection. The wave reflection is created primarily by impedance mismatch at the branch points of the arterial system with very small resistance from the arterioles.1) There are many reflection points in the body at various distances from the heart. However, reflected waves act like a single wave arising from one functional reflection point. The velocity of the traveling pressure wave is influenced by the stiffness of the arteries. In elastic arteries, the PWV is low. Normally, the reflected wave arrives at the aortic root during diastole and augments the coronary circulation. However, with increased stiffness of the aorta there is an increase in the aortic PWV; in this case the reflected wave returns earlier at the aortic root, during late systole, when the ventricle is still ejecting blood, adding the reflected wave to the forward wave and augmenting the central systolic pressure. Increase in the central systolic pressure and pulse pressure results in an increase in arterial wall stress, progression of atherosclerosis and the development of left ventricular hypertrophy due to the increased left ventricular afterload.41) The early return of the reflected wave also causes a decrease in the central diastolic pressure; this results in the reduction of the coronary artery perfusion pressure.1) The augmentation index (AIx) is a commonly used and simple method to measure the effects of wave reflection.
AIx is calculated as follows: AIx (%)=(ΔP/PP)×100, where ΔP is the pressure difference between the peak systolic pressure and an early inflection point that indicates the beginning upstroke of the reflected pressure wave, and PP is the pulse pressure.
The second systolic peak is the shoulder of the arterial wave and the first peak is the peak of the central systolic pressure (Fig. 4). To define the effects of the reflected wave on the left ventricle and coronary artery, the AIx should be measured from the pressure waveform of the central arteries, i.e. the ascending aorta. However, the direct recording of the pressure waveform, of the ascending aorta, is invasive and not practical in the clinical setting. More recently, the aortic pressure waveform has been derived from the pressure waveform obtained from the superficial arteries, i.e. radial or carotid arteries, using the transfer function.42)43) The pressure waveform from the radial artery is recorded non-invasively with applanation tonometry, and then the aortic pressure waveform is derived by a generalized transfer function (SphygmoCor, AtCor, Sydney, Australia).9) Usually the recording of the pressure waveform from the radial artery is preferred because the radial artery is well supported by bone, allowing an optimal pressure waveform to be recorded. Recording of a pressure waveform from the carotid artery is much more difficult and requires highly trained expertise, although a transfer function is not necessary, since the carotid artery is very close to aorta and the waveforms are similar; these measurements are complicated by obesity and carotid stenosis. Although the use of the aortic AIx derived from the radial pressure waveform is commonly used as an indicator of aortic stiffness, issues regarding the accuracy of the generalized transfer function have been raised.44) Some investigators have reported no relationship between the central aortic AIx and the aortic PWV, and have suggested that the aortic AIx and PWV cannot be used interchangeably as an index of aortic stiffness.45) Although it remains to be confirmed, the differences may be due to inaccuracies derived from the central pressure waveform using the generalized transfer function from the radial artery.46)
Reconstruction of the central aortic waveform from the peripheral arteries using the generalized transfer function can also estimate the central aortic pressure from the brachial blood pressure. The estimation of the central aortic blood pressure using the transfer function has been validated for its accuracy.42) The conditions influencing AIx are age47) and blood pressure.48) AIx is also higher in patients with type I diabetes49) and hypercholesterolemia.50) Although data on the value of AIx for the prediction of cardiovascular outcomes is limited, the AIx and central pulse pressure have been shown to be independent predictive values for all-cause and cardiovascular mortality in patients with end-stage renal disease,51)52) in addition to death, myocardial infarction and clinical restenosis in patients undergoing coronary intervention.53)
In the CAFÉ substudy of the ASCOT, there was no difference in aortic PWV between amlodipine and atenolol treatment groups. However, a difference in the central aortic pulse pressure, derived from the brachial artery, suggested that a difference in clinical outcomes between the two treatment groups might be explained by improvement of the wave reflection as a potential mechanism.54)
The digital volume pulse wave has a characteristic incisura or point of inflection (IP) during the down-slope phase. The IP has the tendency to increase with generalized systemic vasoconstriction.55) In patients with hypertension and arteriosclerosis, the shape of the digital volume pulse loses the rebound wave and becomes triangular.55) The IP is lowered with nitrates in a dose dependent manner, and can be used for the evaluation of endothelial function.56) The digital volume pulse waveform can be obtained from the finger with infrared photoplethysmography.
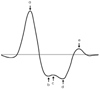
Four waves in systole (a, b, c, and d) and one wave in diastole (e) are obtained from the second derivative of the pressure waveform.57) The ratio of the height of the d wave and that of the a wave (d/a) have been related to age and arterial blood pressure.57) The b/a ratio increases with age, c/a, d/a and e/a ratios decrease with age.57) From these results, the second derivative aging index is defined as follows (Fig. 5):
Aging index=(b-c-d-e)/a. However, the aortic PWV was reported to be a better marker for the presence of atherosclerotic alterations than the "Aging index".58) Recently, the "Compliance index" derived from the digital volume pulse waveform was proposed; however, it showed lower values in patients with risk factors.59) Although measurement of the digital volume pulse wave is easy, portable and useful for epidemiology studies, further investigation is needed for validation of various indices, such as its relationship with central aortic stiffness and predictability of cardiovascular risk.
Ambulatory blood pressure monitoring is commonly used in clinical practice. The 24 hour recording of ambulatory blood pressure allows for the calculation of the regression slope of the diastolic pressure on the systolic blood pressure. The definition of the ambulatory arterial stiffness index (AASI) is as follow:
AASI=1-(the regression slope of diastolic pressure on systolic blood pressure). AASI is significantly correlated with PWV (r=0.51) and the central AIx (r=0.48). The Dublin Outcome Study showed that the AASI was a stronger predictor than the pulse pressure for fatal stroke in those with ambulatory normotension compared to patients with hypertension,60) in the general population.61) However, the AASI was not independently related to the carotid-femoral PWV by the multiple linear regression analysis, and was influenced by nocturnal blood pressure reduction.62) Moreover, the predictive value of the AASI for cardiovascular mortality and stroke was lower than that reported with the aortic PWV.3)60) Therefore, further studies are needed to validate the usefulness of the AASI as a surrogate index for arterial stiffness.
In addition to the above mentioned methods, investigations of additional novel approaches are ongoing for use in the clinical setting.63)
Arterial stiffness is a useful measure for the prediction of cardiovascular risk in the general population, patients with hypertension, diabetes, and end-stage renal disease. A number of methods can be used to measure arterial stiffness noninvasively at low cost that are easily applied in the clinical setting. Clinicians or investigators must choose the method that is appropriate for clinical application and/or research. Thus, the knowledge of the advantages and limitations of each method is essential for accurate and reproducible measurement of arterial stiffness.
Figures and Tables
Fig. 1
Measurement of carotid-femoral pulse wave velocity (PWV). Carotid and femoral pulse waves were obtained with pressure sensitive transducers attached over the carotid and femoral arteries. The pulse transit time (Δt) is the time interval between the onset of the carotid and femoral pulse wave upstroke (foot-to-foot method). The pulse wave travel distance (D), between the carotid and femoral pulse wave recording point, is measured over the body surface with a tape measure.
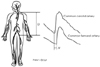
Fig. 2
Measurement of arterial diameter with ultrasound. Radiofrequency tracking (eTRACKING) enables automatic edge detection of the arterial wall movements from the M-mode image.

Fig. 3
Measurement of systemic arterial compliance with the area method. Area (Ad) is computed from end-systole to end-diastole, i.e. area under the diastolic decay portion of the obtained pulse pressure contour. Ps and Pd are end-systolic and end-diastolic pressures.
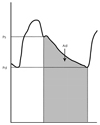
Fig. 4
Measurement of the augmentation index. Pressure waveform obtained in the ascending aorta. Augmentation index is calculated as the pressure difference between the peak systolic pressure and an early inflection point that indicates the beginning upstroke of the reflected pressure wave (ΔP), expressed as a percentage of the pulse pressure (PP).
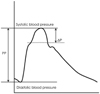
Fig. 5
Measurement of arterial stiffness from digital photophlethysmography. Four waves in systole (a, b, c, and d) and one wave in diastole (e) are obtained from second derivative of pressure waveform. The height of each wave is measured from the baseline and expressed as positive or negative values. The ratios of the heights are expressed as percentage (%) and aging index is defined as (b-c-d-e)/a.
References
1. Nichols WN, O'Rourke MF. Macdonald's Blood Flow In Arteries: Theoretical, Experimental and Clinical Principles. 1998. 4th ed. London: Arnold;201–222.
2. Ohtsuka S, Kakihana M, Watanabe H, Sugishita Y. Chronically decreased aortic distensibility causes deterioration of coronary perfusion during increased left ventricular contraction. J Am Coll Cardiol. 1994. 24:1406–1414.
3. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001. 37:1236–1241.
4. Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999. 99:2434–2439.
5. Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006. 113:664–670.
6. Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Soaciety of Hypertension (ESH) and of the European Society of Cardiology (ESH). J Hypertens. 2007. 25:1105–1187.
7. Safar ME, O'Rourke MF. Arterial Stiffness in Hypertension. 2006. Amsterdam: Elsevier;53–62.
8. Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement: validation and clinical application studies. Hypertension. 1995. 26:485–490.
9. Wilkinson IB, Fuchs SA, Jansen IM, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998. 16:2079–2084.
10. Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002. 25:359–364.
11. Rhee MY, Han SS, Lyu S, Lee MY, Kim YK, Yu SM. Short-term treatment with angiotensin II antagonist in essential hypertension: effects of losartan on left ventricular diastolic function, left ventricular mass, and aortic stiffness. Korean Circ J. 2000. 30:1341–1349.
12. Chiu YC, Arand PW, Shroff SG, Feldman T, Carroll JD. Determination of pulse wave velocities with computerized algorithms. Am Heart J. 1991. 121:1460–1470.
13. Lee YS, Kim KS, Nam CW, Kim YN. Increased arterial stiffness in patients with cardiac syndrome X: pulse wave velocity in cardiac syndrome X. Korean Circ J. 2005. 35:424–428.
14. Rhee MY. Acute and chronic effects of smoking on the arterial wall properties and the hemodynamics in smokers with hypertension. Korean Circ J. 2005. 35:493–499.
15. Rhee MY, Na SH, Kim YK, Lee MM, Kim SK, Kim W. Increased arterial stiffness in Behcet's disease patients. Korean Circ J. 2006. 36:676–682.
16. Matsuoka O, Otsuka K, Murakami S, et al. Arterial stiffness independently predicts cardiovascular events in an elderly community. Biomed Pharmacother. 2005. 59:Suppl 1. S40–S44.
17. Tomiyama H, Koji Y, Yambe M, et al. Brachial-ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J. 2005. 69:815–822.
18. Laurent S, Cockcroft J, van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006. 27:2588–2605.
19. Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001. 21:2046–2050.
20. Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002. 39:1083–1087.
21. Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993. 88:1456–1462.
22. Reneman RS, van Merode T, Hick P, Muytjens AM, Hoeks AP. Age-related changes in carotid artery wall properties in men. Ultrasound Med Biol. 1986. 12:465–471.
23. Riley WA, Barnes RW, Evans GW, Burke GL. Ultrasonic measurement of the elastic modulus of the common carotid artery. Stroke. 1992. 23:952–956.
24. Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction: a noninvasive method to predict severity of coronary atherosclerosis. Circulation. 1989. 80:78–86.
25. Liu ZR, Ting CT, Zhu SX, Yin FC. Aortic compliance in human hypertension. Hypertension. 1989. 14:129–136.
26. Hoeks AP, Brands PJ, Smeets FA, Reneman RS. Assessment of the distensibility of superficial arteries. Ultrasound Med Biol. 1990. 16:121–128.
27. Reneman RS, van Merode T, Hick P, Hoeks AP. Cardiovascular applications of multi-gate pulsed doppler systems. Ultrasound Med Biol. 1986. 12:357–370.
28. Barth JD, Blankenhorn DH, Wickham E, Lai JY, Chin HP, Selzer RH. Quantitative ultrasound pulsation study in human carotid artery disease. Arteriosclerosis. 1988. 8:778–781.
29. Nagai Y, Fleg JL, Kemper MK, Rywik TM, Earley CJ, Metter EJ. Carotid arterial stiffness as a surrogate for aortic stiffness: relationship between carotid artery pressure-strain elastic modulus and aortic pulse wave velocity. Ultrasound Med Biol. 1999. 25:181–188.
30. Rhee BH, Park JH, Kim HS, et al. Increased aortic stiffness is associated with increased left ventricular mass and diastolic dysfunction. Korean Circ J. 2005. 35:525–532.
31. Nichols WN, O'Rourke MF. Macdonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 1998. London: Arnold;170–200.
32. DeBakey ME, Lawrie GM, Glaeser DH. Patterns of atherosclerosis and their surgical significance. Ann Surg. 1985. 201:115–131.
33. Li R, Duncan BB, Metcalf PA, et al. B-mode-detected carotid artery plaque in a general population. Stroke. 1994. 25:2377–2383.
34. Paini A, Boutouyrie P, Calvet D, Tropeano AI, Laloux B, Laurent S. Carotid and aortic stiffness: determinants of discrepancies. Hypertension. 2006. 47:371–376.
35. Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension. 1998. 32:570–574.
36. Mohiaddin RH, Underwood SR, Bogren HG, et al. Regional aortic compliance studied by magnetic resonance imaging: The effects of age, training, and coronary artery disease. Br Heart J. 1989. 62:90–96.
37. Liu Z, Brin KP, Yin FC. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am J Physiol. 1986. 251:H588–H600.
38. de Simone G, Roman MJ, Koren MJ, Mensah GA, Ganau A, Devereux RB. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension. 1999. 33:800–805.
39. Cohn JN, Finkelstein S, McVeigh G, et al. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995. 26:503–508.
40. Manning TS, Shykoff BE, Izzo JL Jr. Validity and reliability of diastolic pulse contour analysis (windkessel model) in humans. Hypertension. 2002. 39:963–968.
41. Nichols WW, O'Rourke MF, Avolio AP, et al. Effects of age on ventricular-vascular coupling. Am J Cardiol. 1985. 55:1179–1184.
42. Chen CH, Ting CT, Nussbacher A, et al. Validation of carotid artery tonometry as a means of estimating augmentation index of ascending aortic pressure. Hypertension. 1996. 27:168–175.
43. Chen CH, Nevo E, Fetics B, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure: validation of generalized transfer function. Circulation. 1997. 95:1827–1836.
44. Lacy PS, O'Brien DG, Stanley AG, Dewar MM, Swales PP, Williams B. Increased pulse wave velocity is not associated with elevated augmentation index in patients with diabetes. J Hypertens. 2004. 22:1937–1944.
45. Sakurai M, Yamakado T, Kurachi H, et al. The relationship between aortic augmentation index and pulse wave velocity: an invasive study. J Hypertens. 2007. 25:391–397.
46. Hope SA, Meredith IT, Tay D, Cameron JD. 'Generalizability' of a radial-aortic transfer function for the derivation of central aortic waveform parameters. J Hypertens. 2007. 25:1812–1820.
47. Kelly R, Hayward C, Avolio A, O'Rourke MF. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989. 80:1652–1659.
48. Wilkinson IB, MacCallum H, Hupperetz PC, van Thoor CJ, Cockcroft JR, Webb DJ. Changes in the derived central pressure waveform and pulse pressure in response to angiotensin II and noradrenaline in man. J Physiol. 2001. 530:541–550.
49. Wilkinson IB, MacCallum H, Rooijmans DF, et al. Increased augmentation index and systolic stress in type 1 diabetes mellitus. QJM. 2000. 93:441–448.
50. Wilkinson IB, Prasad K, Hall IR, et al. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol. 2002. 39:1005–1011.
51. London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001. 38:434–438.
52. Safar ME, Blacher J, Pannier B, et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002. 39:735–738.
53. Weber T, Auer J, O'Rourke MF, et al. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J. 2005. 26:2657–2663.
54. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes. Circulation. 2006. 113:1213–1225.
55. Dillon JB, Hertzman AB. The form of the volume pulse in the finger pad in health, arteriosclerosis, and hypertension. Am Heart J. 1941. 21:172–190.
56. Morikawa Y. Characteristic pulse wave caused by organic nitrates. Nature. 1967. 213:841–842.
57. Takazawa K, Tanaka N, Fujita M, et al. Assessment of vasoactive agents and vascular aging by the second derivative of photoplethysmogram waveform. Hypertension. 1998. 32:365–370.
58. Bortolotto LA, Blacher J, Kondo T, Takazawa K, Safar ME. Assessment of vascular aging and atherosclerosis in hypertensive subjects: second derivative of photoplethysmogram versus pulse wave velocity. Am J Hypertens. 2000. 13:165–171.
59. Chen JY, Tsai WC, Wu MS, et al. Novel compliance index derived from digital volume pulse associated with risk factors and exercise capacity in patients undergoing treadmill exercise tests. J Hypertens. 2007. 25:1894–1899.
60. Dolan E, Thijs L, Li Y, et al. Ambulatory arterial stiffness index as a predictor of cardiovascular mortality in the Dublin Outcome Study. Hypertension. 2006. 47:365–370.
61. Kikuya M, Staessen JA, Ohkubo T, et al. Ambulatory arterial stiffness index and 24-hour ambulatory pulse pressure as predictors of mortality in Ohasama, Japan. Stroke. 2007. 38:1161–1166.
62. Schillaci G, Parati G, Pirro M, et al. Ambulatory arterial stiffness index is not a specific marker of reduced arterial compliance. Hypertension. 2007. 49:986–991.
63. Park SM, Seo HS, Lim HE, et al. Assessment of the arterial stiffness index as a clinical parameter for atherosclerotic coronary artery disease. Korean Circ J. 2004. 34:677–683.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download