Abstract
Nontuberculous mycobacteria (NTM) are emerging pathogens that affect both immunocompromised and immunocompetent patients. The incidence and prevalence of NTM lung disease are increasing worldwide and rapidly becoming a major public health problem. For the diagnosis of NTM lung disease, patients suspected to have NTM lung disease are required to meet all clinical and microbiologic criteria. The development of molecular methods allows the characterization of new species and NTM identification at a subspecies level. Even after the identification of NTM species from respiratory specimens, clinicians should consider the clinical significance of such findings. Besides the limited options, treatment is lengthy and varies by species, and therefore a challenge. Treatment may be complicated by potential toxicity with discouraging outcomes. The decision to start treatment for NTM lung disease is not easy and requires careful individualized analysis of risks and benefits. Clinicians should be alert to those unique aspects of NTM lung disease concerning diagnosis with advanced molecular methods and treatment with limited options. Current recommendations and recent advances for diagnosis and treatment of NTM lung disease are summarized in this article.
Nontuberculous mycobacteria (NTM) are ubiquitous organisms responsible for opportunistic infections with a broad spectrum of virulence. The incidence and prevalence of NTM lung disease continue to increase worldwide, leading to an emerging public health problem123. Although the distribution of NTM species varies markedly based on geography, Mycobacterium avium complex (MAC) is the most common pathogen in most areas followed by M. abscessus complex (MABC) and M. kansasii4.
The American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) published clinical guidelines for NTM in 20075. Because of the difficulty in distinguishing between NTM isolation and disease, clinical and microbiologic criteria are needed for the diagnosis of NTM lung disease. Currently recommended treatment regimens, drug resistance patterns, and treatment outcomes differ according to the NTM species, and management is a lengthy complicated process with limited therapeutic options5. However, the current guidelines rely largely on expert opinion, and some aspects of the recommendations remain controversial67. In addition, adherence to the guidelines for treating NTM lung disease is suboptimal, or potentially harmful antibiotics regimens are commonly prescribed8. Moreover, to date, there have been advances in molecular diagnostic methods for identification of NTM species and drug resistance, and antibiotic treatment of NTM lung disease. Therefore, revised evidence-based guidelines, practical information, and education are needed for practicing clinicians.
In this review, we summarize the recent advances that allow earlier diagnosis, rapid identification of NTM subspecies and improved treatment of NTM lung disease, and recommend a multidisciplinary approach in clinical practice based on updated literature reviews and standard guidelines. This review focuses on the most clinically frequent and significant species of NTM lung diseases in immunocompetent individuals.
The isolation of NTM remains a clinical dilemma for clinicians. Because NTM exists naturally in the environment, isolation of NTM from a nonsterile respiratory specimen does not mean they are causative organisms of lung disease9. Diagnosis of NTM lung disease requires the clinician to integrate clinical, radiographic, and microbiological data, but, ultimately, diagnosis can be confirmed by (1) at least two positive cultures from sputum, (2) one positive culture in the case of bronchoscopic wash or lavage, or (3) a transbronchial or other lung biopsy with a positive culture for NTM or compatible histopathological features such as granulomatous inflammation or stainable acid-fast bacilli (AFB), and one positive sputum or bronchial wash culture for NTM regardless of the mycobacterial strain5. Therefore, symptomatic patients with compatible radiographic findings should meet the microbiological criteria in order to establish a diagnosis of NTM lung disease5.
Diagnosis of NTM lung disease requires considerable time due to its slow growth, and may be misdiagnosed as tuberculosis (TB) or other AFB-positive bacilli10. These factors and a low index of clinical suspicion often result in delayed diagnosis. The symptoms are often nonspecific such as chronic cough, increased sputum production, dyspnea, low-grade fever, malaise and weight loss, and overlapping clinical characteristics with pulmonary TB1112.
Radiological imaging is important when NTM lung disease is suspected. The broad range of radiological patterns seen in NTM lung disease includes bronchiectasis, nodular lesions, cavitary lesions, and parenchymal consolidation13. NTM lung disease has two major manifestations: fibrocavitary and nodular bronchiectatic forms5. The fibrocavitary form resembles pulmonary TB and typically affects elderly men with underlying lung disease. This form is characterized by cavities with areas of increased opacity, usually located in the upper lobes (Figure 1). Pleural thickening and volume loss by fibrosis with traction bronchiectasis are frequent. Cavitation is the most typical radiologic feature in pulmonary TB; however, NTM lung disease tends to cause thin-walled cavities, often involving pleura without lymph node calcification, no atelectasis and usually progresses more slowly than pulmonary TB1415. The nodular bronchiectatic form shows bilateral, multilobar bronchiectasis, especially in the middle and lower lung fields, with small nodules on chest radiography and high resolution computed tomography (HRCT) (Figure 2)1617. This pattern of NTM lung disease occurs predominantly in elderly nonsmoking women without underlying lung disease, and appears more commonly in those with a thin body habitus1819. There is evidence for a possible role of NTM infection causing bronchiectasis. On the other hand, bronchiectasis can precede NTM infection in some conditions20. A recent meta-analysis showed that the overall prevalence of NTM infection was 9.3% in patients with bronchiectasis21. Clinicians should be aware that bronchiectasis and NTM lung disease are connected. Because of considerable overlap in common HRCT findings, it is difficult to differentiate species of NTM lung disease based on radiologic patterns2223.
Because NTM are present in the environment, especially in water sources, the careful collection of high-quality respiratory specimens is necessary to avoid contamination. Moreover, the temporary presence of NTM species from environmental sources in the airway may lead to positive samples924. Therefore, collection of three early-morning specimens on different days is preferred for diagnosis of NTM lung disease5. Induction of sputum with hypertonic saline may be used in patients who are unable to produce sputum spontaneously. Two types of AFB stains are commonly used: the carbol fuchsin stain (Ziehl-Neelsen or Kinyoun method) and the fluorochrome procedure (auramine O alone or in combination with rhodamine B). Kinyoun's method appears inferior to both the Ziehl-Neelsen and fluorochrome methods2526.
AFB staining cannot differentiate between M. tuberculosis and NTM. Nucleic acid amplification (NAA) tests for the detection of M. tuberculosis are needed. Several commercial tests such as the Xpert MTB/RIF assay (Xpert assay; Cepheid, Sunnyvale, CA, USA), the Cobas TaqMan MTB test (Roche Diagnostics, Rotkreuz, Switzerland), and the Amplified M. tuberculosis direct test (Hologic Inc., San Diego, CA, USA) are widely used. Compared with AFB smear microscopy, NAA testing has a greater positive predictive value (>95%) for M. tuberculosis with AFB smear-positive specimens in settings in which NTMs are common27.
Culture remains the gold standard for laboratory confirmation of NTM and is required for genotypic identification and drug susceptibility tests (DST). The culture media are similar to that used for M. tuberculosis. Solid media include either egg-based media, such as Löwenstein-Jensen agar, or agarbased media such as Middlebrook 7H10 and 7H11 media. Cultures on solid media allow for the observation of colony morphology, growth rates, species categorization based on pigmentation, and quantitation of the infecting organism. Solid media also serves as a backup when liquid media cultures are contaminated25. Liquid systems are more sensitive and reduce the delay in the detection of NTM but are prone to contamination by other microorganisms and bacterial overgrowth25. Therefore, all cultures for mycobacteria should include both solid and liquid media for the detection and enhancement of growth, which was shown to increase the sensitivity of NTM detection by an average of 15%9. An incubator system of liquid culture, which contains enriched Middlebrook 7H9 broth, can automatically detect the growth of mycobacteria including NTM in the laboratory. Additionally, there are various commercially available automated culture-reading systems28.
Since treatments and outcomes differ depending on the NTM species, NTM identification is clinically important. Traditional biochemical tests or high performance liquid chromatography for NTM identification have been replaced by molecular methods such as line probe hybridization, polymerase chain reaction (PCR)-restriction fragment length polymorphism analysis, real-time PCR, and DNA sequencing. Some commercial kits are available, including the AccuProbe system (Hologic Inc.), INNO-LiPA Mycobacteria system (Fujirebio Europe, Ghent, Belgium), and GenoType Mycobacterium system (Hain Lifescience, Nehren, Germany)29.
Gene sequencing is the reference method for the identification of NTM species and may be performed for uncommonly encountered species or precise identification at the subspecies level. Sequencing of the 16S rRNA gene allows discrimination at the species level or to the complex level such as MABC. However, single-target sequencing cannot be used to accurately differentiate species30, and for a higher level of discrimination up to the subspecies level, gene sequencing of several targets using key genes, such as hsp65 and rpoB , and the 16S–23S internal transcribed spacer, is needed3132333435.
The taxonomy of rapidly growing mycobacteria (RGM), especially MABC, has frequently been revised in recent years, leading to considerable confusion among clinicians3637. Based on whole genome sequencing data, MABC can be divided into at least three close subspecies (subsp.) with regards to the erythromycin ribosomal methyltransferase gene (41) or erm(41) sequence: M. abscessus subsp. abscessus (hereafter M. abscessus), M. abscessus subsp. massiliense (hereafter M. massiliense), and M. abscessus subsp. bolletii (hereafter M. bolletii)3839.
A recent major advance in MABC was the discovery of inducible macrolide resistance, in which the organism develops resistance to the macrolides in vitro after prolonged incubation (susceptible at day 3, but resistant at day 14), or by preincubation in macrolide-containing media40. The erm(41) gene, encoding a methyltransferase that methylates the site of action of macrolides at the 23S rRNA level, is present in several species in RGM, and confers natural inducible resistance to macrolides.
In addition, M. abscessus strains have a T or C polymorphism at the 28th nucleotide on erm(41): T28 strains demonstrate inducible clarithromycin resistance, while C28 strains are susceptible4142. M. bolletii strains have erm(41) sequences similar to the sequence of the T28 M. abscessus group, associated with inducible clarithromycin resistance41. M. abscessus possesses a novel erm(41) gene and the production of Erm41 methyltransferase is predictive of clinical failure with clarithromycin 40. M. massiliense has a dysfunctional erm gene and exhibits susceptibility to macrolides4143.
To date, gene sequencing is the most accurate method for identifying NTM species; however, species-level discrimination may require analysis of several genes and has been limited to specialized laboratories. In recent years, the matrixassociated laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) method is increasingly being applied clinically for bacterial and fungal infections, and its utility for the identification of NTM has emerged as a potential tool for NTM identification in a limited scope. The MALDI-TOF MS method is a method of identifying target bacterial species by comparing the mass spectral patterns of molecules, mainly ribosomal proteins, specific to NTM species, in a library of known NTM strains with unquestionable reliability and cost-effectiveness44. In recent comparative studies of MALDI-TOF MS and conventional real-time PCR methods, the GenoType Mycobacterium system or 16S rRNA gene sequencing for the evaluation of NTM suggested that MALDI-TOF MS is a valuable tool for the identification of these groups of organisms454647. MALDI-TOF was found to be an accurate, rapid, and cost-effective system for identification of NTM species. However, MALDI-TOF MS requires a moderate amount of organism, unlike sequencing where only scant growth is needed45. Furthermore, the discrimination power of MALDI-TOF MS largely depends on the quality of the databases and cannot accurately differentiate MABC to a subspecies level. Therefore, further study is required to validate these results in clinical practice945.
The role of a DST is to guide the design of optimal treatment regimens. However, the DST for NTM is difficult and controversial because of discrepancies between in vitro and in vivo clinical outcomes, with the exception of macrolides and amikacin48. Among slow-growing mycobacteria (SGM), clear correlations have been established for macrolides and amikacin in MAC lung disease and for rifampicin in M. kansasii lung disease. Macrolide resistance in MAC is caused by a mutation in the 23S rRNA gene macrolide binding site, usually selected due to macrolide monotherapy49. Only routine macrolide susceptibility testing for all MAC isolates is advised and clarithromycin is the preferred class representative according to the Clinical and Laboratory Standards Institute50. In the case of macrolide resistance, moxifloxacin and linezolid should be tested.
Given that treatment failure is associated with rifampin resistance and drug treatment histories are generally unavailable during laboratory procedures, rifampin and clarithromycin are the currently recommended drugs for primary susceptibility testing for M. kansasii5. M. kansasii isolates resistant to rifampin should be tested against a panel of secondary agents, including rifabutin, ethambutol, isoniazid, clarithromycin, fluoroquinolones, amikacin, and sulfonamides5. For RGM, agents that should be tested are amikacin, cefoxitin, ciprofloxacin, clarithromycin, doxycycline (or minocycline), imipenem, linezolid, moxifloxacin, trimethoprim-sulfamethoxazole, and tobramycin50. In addition, it is recommended that the final reading for clarithromycin be at least 14 days, unless resistance is recognized earlier, to detect inducible macrolide resistance in RGM, especially M. abscessus50.
The management of NTM lung disease is a challenge that should be undertaken by experienced clinicians at centers equipped with reliable laboratory services for mycobacterial cultures and in vitro DST as it the requires prolonged use of costly combinations of multiple drugs with a significant potential for toxicity. The diagnosis of NTM lung disease does not obligate the initiation of therapy against NTM species, and a decision must be made based on the potential risks and benefits of therapy for individual patients5. Unlike pulmonary TB, clinicians may observe patients with minimal symptoms and stable radiographic disease closely without invasive workups or treatment, provided the patients do not have decreased host immunity towards NTM and avoid aggravating factors through education. However, once the clinician decides to start treatment, the goal of curative therapy in NTM lung disease is 12 months of culture negativity, and therefore, frequent sputum sampling every 1–2 months is needed5. Simultaneously, clinicians should consider quality of life and a patientcentered approach rather than solely expecting microbiologic erradication51.
Macrolides (clarithromycin and azithromycin) are the cornerstones of treatment for MAC lung disease52. The standard optional regimen includes a rifamycin (rifampin or rifabutin), ethambutol, and a macrolide administered for 18–24 months, including 12 months of sputum culture negativity5. Considering potential toxicity, intolerance, and costs of daily therapy with macrolide-based regimens, current guidelines recommended three-times-weekly intermittent therapy with a macrolide (clarithromycin 1,000 mg or azithromycin 500 mg), rifampin 600 mg, and ethambutol at 25 mg/kg for the initial treatment of non-cavitary nodular bronchiectasis NTM lung disease5. Recent studies have shown that intermittent thriceweekly therapy is effective and better tolerated compared with daily therapy in non-cavitary nodular bronchiectatic MAC lung disease5354. There were no differences in response between clarithromycin and azithromycin regimens53. However, for patients with fibrocavitary forms or cavitary nodular bronchiectatic disease, daily therapy is recommended with clarithromycin (1,000 mg/day) or azithromycin (250 mg/day), rifampin (10 mg/kg/day, maximum 600 mg/day) or rifabutin (150–300 mg/day), and ethambutol (15 mg/kg/day), and amikacin or streptomycin could be added for the first 2 or 3 months of therapy in severe disease5.
For patients failing standard therapy, the addition of moxifloxacin to multidrug regimens may improve treatment outcomes, but there has been little evidence to support the use of these regimens5556. With the requirement of prolonged treatment of aminoglycosides in cases of refractory MAC lung disease, inhaled amikacin can be used as an alternative to parenteral use, even with limited evidence57. Clofazimine can be used as an effective alternative to rifamycins or in refractory MAC disease585960. Ultimately, optimal therapeutic attempts and avoidance of the emergence of macrolide-resistant MAC strains is critical for successful treatment of MAC lung disease.
M. kansasii remains a relatively easily treatable pathogen in NTM lung disease and often has a similar presentation to that of pulmonary TB with fibrocavitary lesions in the upper lobes5. M. kansasii is sensitive to standard anti-TB drugs except for pyrazinamide, and there is good correlation between in vitro and in vivo susceptibility, especially for rifampicin48. The recommended regimen for treating M. kansasii lung disease includes daily isoniazid 300 mg, rifampicin 600 mg, and ethambutol 15 mg/kg for 12 months after sputum culture conversion5. Recently, a favorable experience with a low relapse rate in a 12-month fixed-course treatment regimen, including rifampin, isoniazid, and ethambutol, supplemented with streptomycin during the first 2–3 months, was reported61. Because of the excellent activity of the macrolides for M. kansasii , a macrolide-containing regimen has also been suggested626364.
For the treatment of MABC lung disease, the strategy should be individualized based on in vitro DST results and patient tolerance. The therapy for M. abscessus lung disease remains a difficult problem and more clinical trials are needed because of the poor outcomes and paucity of treatment success65. Current guidelines suggest an oral macrolide with two parenteral agents for several months to symptomatically improve and slow down the disease progression for M. abscessus lung disease5. The most active parenteral agents include amikacin (10–15 mg/kg/day or 15–25 mg/kg thrice weekly), cefoxitin (200 mg/kg/day up to 12 g/day in divided doses), imipenem (500–1,000 mg2 to four times daily), and tigecycline (50 mg once or twice daily)65. The role of inhaled amikacin has yet to be defined57. Although more than half of the M. abscessus isolates are moderately susceptible to moxifloxacin in some studies6667, the results are controversial68. Moreover, regarding the bactericidal effects of amikacin and moxifloxacin against M. abscessus , none of these drugs showed bactericidal activity697071. Considering drug toxicity and inconvenience, aggressive parenteral therapy was suggested for 2–4 months, followed by macrolide therapy with additional oral agents such as a fluoroquinolone, linezolid, clofazimine, or inhaled amikacin as a step-down therapy65.
In a recent comparative study between clarithromycin and azithromycin, clarithromycin induced greater erm(41) expression with higher macrolide resistance than azithromycin in M. abscessus infection43. However, more recent studies do not support the suggestion of preferential use of azithromycin over clarithromycin72. Therefore, the choice of macrolide depends on tolerability. M. massiliense isolates do not show inducible resistance to macrolides, and treatment outcomes showed favorable responses in M. massiliense lung disease737475. The inducible macrolide resistance may explain disappointing treatment responses to macrolide-based regimens against M. abscessus lung disease. Because reliably effective medical therapy for M. abscessus lung disease remains elusive, surgical resection should be considered, especially in focal disease76.
Multiple drug therapy in NTM lung disease can cause adverse effects, which leads to treatment discontinuation or patient nonadherence52. Current guidelines recommend monitoring for drug toxicity at repeat intervals5. Gastrointestinal side effects with oral agents are common. Due to severe gastrointestinal disturbances, the use of macrolides may require dose adjustment5. Drug-induced hepatotoxicity due to rifampin, macrolides, imipenem, or tigecycline should be monitored by liver function tests, and monitoring complete blood count when using rifampin, imipenem, or tigecycline is recommended due to hematologic disturbances such as leukopenia or thrombocytopenia52. Renal function testing is also needed, especially in aminoglycosides52. Due to the risk of ototoxicity such as hearing loss, tinnitus, or vestibular toxicity, patients who receive streptomycin or amikacin should be educated regarding the signs and symptoms of toxicity with audiometry testing at the start of therapy and again on subsequent visits with discontinuation, or a decrease in dosage or frequency if signs suggestive of toxicity occur52. Macrolides can also cause ototoxicity or vestibular dysfunction52. Because optic neuritis and peripheral neuropathy are important side effects with ethambutol and linezolid, patients should be tested at baseline and periodically during treatment52. Patients should visit clinicians immediately for ototoxicity or optic neuritis-related symptoms.
Clinicians should consider drug-drug interaction following comorbidities and associated concomitant therapies, especially in elderly patients with NTM lung disease77. Concomitant use of rifampin often leads to reduced peak serum levels for macrolides and moxifloxacin, which could partially explain the poor outcomes of currently recommended treatment regimens7879. Concomitant use of macrolides and warfarin or new oral anticoagulants can increase bleeding risk80. Rifamycins may decrease the anticoagulative effect of warfarin because rifamycins are strong inducers of the cytochrome P-450 enzyme52. However, clarithromycin is both a substrate for and an inhibitor of cytochrome P 3A enzymes, whereas azithromycin is not. Thus, azithromycin is often preferred, in order to avoid drug interactions, including interactions with rifamycins52. As a result, the medication list of patients with NTM lung disease should be reviewed before starting antimicrobial therapy, and potential drug-drug interactions should be monitored. Especially in patients receiving multiple medications for comorbidities, gradual introduction at 1 to 2 weekly intervals is advisable in order to evaluate tolerance to each medication and medication dose.
Amikacin is an effective drug against most NTM species, but daily or intermittent use of systemic amikacin can have undesirable adverse effects such as ototoxicity and nephrotoxicity52. The current guidelines recommend only systemic use of aminoglycosides5, but recent data have shown that inhaled amikacin is effective in the treatment of refractory NTM lung disease with less toxicity than systemic amikacin57. Although these data are based on a small sample size and retrospective analysis, amikacin inhalation treatment could overcome the side effects of systemic use and could be effective as an adjunctive therapy for treatment of NTM lung disease79. Liposomal amikacin for inhalation could be more effective than free amikacin in eliminating NTM species in vitro and in animal studies81. A multicenter, randomized, double blind, placebo-controlled phase 2 study of liposomal amikacin for inhalation treatment of refractory NTM lung disease has been recently completed (http://www.clinicaltrials.gov;identifier:NCT01315236).
Linezolid, the first oxazolidinone antibacterial agent approved for clinical use, has excellent potential against multidrug-resistant (MDR) TB and some species of NTM, and the oral agent is completely absorbed with near 100% bioavailibility52. However, the clinical use of linezolid for treatment of NTM lung disease has been limited because of the lack of long-term safety data, concern over limiting adverse hematologic events, and cost82.
Tigecyline is active against many gram-positive and -negative organisms in skin and soft tissue infections as a parenteral therapy and shows good in vitro activity against M. abscessus83. A recent study has shown a favorable response to tigecyline salvage treatment in patients with M. abscessus and M. chelonae infections as part of multidrug regimens84. However, due to significant nausea and vomiting, reduced dosage adjustment from 100 to 50 mg per day may be needed for tolerability84. Combined with clarithromycin, tigecycline has high synergistic activity against RGM, but should be used with caution in combination with amikacin because of antagonistic activity with low synergistic activity85.
Clofazimine is an anti-leprosy drug that has been used occasionally in the treatment of MDR-TB and NTM infections52. Clofazimine is administered orally, most often at doses of 100 mg daily52. Common adverse effects with clofazimine are discoloration and dryness of skin, photosensitivity, and gastrointestinal problems86. To date, clofazimine has been difficult to obtain because it is a non-commercial drug, and there are few clinical data regarding clofazimine use for salvage therapy of NTM infection56, but clofazimine and amikacin show significant synergistic activity against both SGM and RGM5887.
Bedaquiline is an oral antimycobacterial agent, recommended for MDR-TB, and showed potential promise for advanced NTM lung disease in a recent small preliminary report88. A total of 10 patients with failed NTM lung disease treatment completed 6 months of bedaquiline therapy, and 60% had a microbiologic response while 90% showed symptom improvement. There were no severe complications including QT prolongation88.
Biofilms are microcolonies of bacteria embedded in the extracellular matrix that provide stability and resistance to human immune mechanisms89. In recent years, some species of NTM have been shown to form biofilms that enhance resistance to disinfectants and antimicrobial agents9091. In clinical practice, poor drug penetration and resistance due to biofilm formation of the respiratory tract is an important barrier to treating NTM lung disease. Moreover, lack of correlation between in vitro and in vivo susceptibility could be related to biofilms. A biofilm is mainly composed of extracellular DNA, proteins and polysaccharides, and extracellular DNA plays a major role in resistance; destruction of extracellular DNA in vitro was found to increase the efficacy of antimicrobial agents92. Therefore, a combination of DNase with antimicrobial agents could be a more effective treatment strategy for future treatment of NTM infection within biofilm formation93.
Except for M. kansasii, NTM lung disease is difficult to control with antimicrobial therapy alone94. Although the role of adjunctive surgical approaches remains unclear, surgery can be effective in cases of significant drug resistance or failure of medical treatment. In the case of localized lesions, appropriate surgical treatment could improve the rate of treatment success in patients with NTM lung disease. To slow disease progress, debulking surgery of the worst area of the destroyed lung may be indicated in selected patients. Additionally, in terms of symptom control such as massive hemoptysis, surgery may be helpful95.
Successful treatment outcomes with sputum conversion can be achieved in 81%–100% of patients after adjuvant surgical resection969798. Despite the favorable treatment success rate, postoperative complications were not uncommon and recent data showed 7%–25% morbidity and 0%–3% mortality969798. Therefore, clinicians should carefully select patients for surgery after discussion in a multidisciplinary setting and comprehensive preoperative evaluation is needed95. Moreover, surgery without combination antimicrobial therapy could not achieve acceptable results. It is important to maintain perioperative antimicrobial therapy throughout the postoperative period95. The optimal duration of antimicrobial therapy before and after surgery remains a topic of debate969798. However, patients are considered to have failed treatment without response after 6 months of appropriate medical therapy or without sputum conversion after 12 months of appropriate therapy99. Therefore, clinicians should consider the potential role of surgery in these cases100. Additionally, even in focal disease, aggressive medical therapy of at least 2 months should be performed before surgery65. Moreover, current guidelines recommend that medical therapy should be continued until the patient has persistently negative sputum cultures for 12 months while undergoing treatments, including surgery5.
The prevalence of NTM lung disease is increasing worldwide, even in immunocompetent individuals. NTM lung disease is becoming a greater public health problem and the financial costs are substantial, particularly in elderly patients. Because NTM is a ubiquitous pathogen, isolation from a respiratory specimen does not necessarily indicate NTM lung disease. Clinical, microbiologic, and radiographic criteria should all be met to make a diagnosis of NTM lung disease. Treatment regimen and response rates differ according to NTM species; therefore, molecular methods for identification of NTM species and DST for optimal treatment regimens are ultimately needed. The diagnosis of NTM lung disease depends on meeting established diagnostic criteria; however, treatment decisions are difficult and still require considerable clinical judgment. Management of NTM lung disease is mainly carried out by medical therapy, a lengthy, expensive, and time-consuming process. Macrolides remain the most effective agents available against SGM and some RGM. Multiple drug therapy with a macrolide, ethambutol and a rifamycin is recommended, and an initial 2–3 months of aminoglycosides may be needed depending on the disease severity of MAC lung disease. Although optimal therapeutic regiments have yet to be established and effective agents are lacking, with frequent side effects in MABC lung disease, treatment with a macrolide and two parenteral agents (amikacin plus cefoxitin or imipenem) has shown favorable outcomes in species of M. abscessus erm(41) C28 sequevar or M. massiliense species with nonfunctional erm(41) gene. There is a lack of evidence and few randomized clinical trials to guide the management for refractory NTM lung disease, macrolide-resistant NTM, or M. abscessus erm(41) T28 sequevar with active erm(41) gene, resulting in inducible resistance to macrolides. However, multiple combination regimens with inhaled amikacin following initial treatment with parenteral aminoglycosides, tigecycline and other promising oral antibiotics such as linezolid, clofazimine, and bedaquiline, and surgical intervention in selected cases have shown promising results. A multidisciplinary approach is important in the diagnosis and treatment of NTM lung disease and improved management of NTM lung disease allows for more comprehensive care. Newer antimicrobial agents and clinical trials are needed in order to improve patient management.
Figures and Tables
Figure 1
The fibrocavitary form of Mycobacterium intracellulare pulmonary disease in a 73-year-old male patient. Chest computed tomography shows a large cavity in the right upper lobe. Note the emphysema in both lungs.
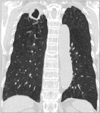
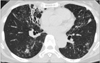
Figure 2
The nodular bronchiectatic form of Mycobacterium intracellulare pulmonary disease in a 70-year-old female patient. Chest computed tomography shows severe bronchiectasis in the right middle lobe and the lingular segment of the left upper lobe. Note the multiple small nodules and tree-in-bud appearances suggesting bronchiolitis in both lungs.
Acknowledgements
The authors would like to thank Dr. Hee Jae Huh (Department of Laboratory Medicine and Genetics, Samsung Medical Center, Seoul, Korea) for providing valuable comments on this manuscript.
References
1. Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013; 34:87–94.
2. Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015; 36:13–34.
3. Koh WJ, Chang B, Jeong BH, Jeon K, Kim SY, Lee NY, et al. Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in South Korea. Tuberc Respir Dis. 2013; 75:199–204.
4. Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTMNET collaborative study. Eur Respir J. 2013; 42:1604–1613.
5. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416.
6. Marras TK, Prevots DR, Jamieson FB, Winthrop KL. Pulmonary MAC Outcomes Group. Opinions differ by expertise in Mycobacterium avium complex disease. Ann Am Thorac Soc. 2014; 11:17–22.
7. Marras TK, Prevots DR, Jamieson FB, Winthrop KL. Pulmonary MAC Outcomes Group. Variable agreement among experts regarding Mycobacterium avium complex lung disease. Respirology. 2015; 20:348–351.
8. Adjemian J, Prevots DR, Gallagher J, Heap K, Gupta R, Griffith D. Lack of adherence to evidence-based treatment guidelines for nontuberculous mycobacterial lung disease. Ann Am Thorac Soc. 2014; 11:9–16.
9. van Ingen J. Microbiological diagnosis of nontuberculous mycobacterial pulmonary disease. Clin Chest Med. 2015; 36:43–54.
10. Kwon YS, Koh WJ. Diagnosis of pulmonary tuberculosis and nontuberculous mycobacterial lung disease in Korea. Tuberc Respir Dis. 2014; 77:1–5.
11. Koh WJ, Yu CM, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Pulmonary TB and NTM lung disease: comparison of characteristics in patients with AFB smear-positive sputum. Int J Tuberc Lung Dis. 2006; 10:1001–1007.
12. Kim YK, Hahn S, Uh Y, Im DJ, Lim YL, Choi HK, et al. Comparable characteristics of tuberculous and non-tuberculous mycobacterial cavitary lung diseases. Int J Tuberc Lung Dis. 2014; 18:725–729.
13. Jeong YJ, Lee KS, Koh WJ, Han J, Kim TS, Kwon OJ. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology. 2004; 231:880–886.
14. Yuan MK, Chang CY, Tsai PH, Lee YM, Huang JW, Chang SC. Comparative chest computed tomography findings of non-tuberculous mycobacterial lung diseases and pulmonary tuberculosis in patients with acid fast bacilli smearpositive sputum. BMC Pulm Med. 2014; 14:65.
15. Chu HQ, Li B, Zhao L, Huang DD, Zhang ZM, Xu JF, et al. Chest imaging comparison between non-tuberculous and tuberculosis mycobacteria in sputum acid fast bacilli smearpositive patients. Eur Rev Med Pharmacol Sci. 2015; 19:2429–2439.
16. Koh WJ, Lee KS, Kwon OJ, Jeong YJ, Kwak SH, Kim TS. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005; 235:282–288.
17. Lee G, Lee KS, Moon JW, Koh WJ, Jeong BH, Jeong YJ, et al. Nodular bronchiectatic Mycobacterium avium complex pulmonary disease: natural course on serial computed tomographic scans. Ann Am Thorac Soc. 2013; 10:299–306.
18. Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008; 178:1066–1074.
19. Kartalija M, Ovrutsky AR, Bryan CL, Pott GB, Fantuzzi G, Thomas J, et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med. 2013; 187:197–205.
20. Park IK, Olivier KN. Nontuberculous mycobacteria in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med. 2015; 36:217–224.
21. Chu H, Zhao L, Xiao H, Zhang Z, Zhang J, Gui T, et al. Prevalence of nontuberculous mycobacteria in patients with bronchiectasis: a meta-analysis. Arch Med Sci. 2014; 10:661–668.
22. Hollings NP, Wells AU, Wilson R, Hansell DM. Comparative appearances of non-tuberculous mycobacteria species: a CT study. Eur Radiol. 2002; 12:2211–2217.
23. Chung MJ, Lee KS, Koh WJ, Lee JH, Kim TS, Kwon OJ, et al. Thin-section CT findings of nontuberculous mycobacterial pulmonary diseases: comparison between Mycobacterium avium-intracellulare complex and Mycobacterium abscessus infection. J Korean Med Sci. 2005; 20:777–783.
24. van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013; 34:103–109.
25. Pfyffer GE. Mycobacterium: general characteristics, laboratory detection, and staining procedures. In : Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, editors. Manual of clinical microbiology. 11th ed. Washington,DC: ASM Press;2015. p. 543–572.
26. Somoskövi A, Hotaling JE, Fitzgerald M, O'Donnell D, Parsons LM, Salfinger M. Lessons from a proficiency testing event for acid-fast microscopy. Chest. 2001; 120:250–257.
27. Huh HJ, Koh WJ, Song DJ, Ki CS, Lee NY. Evaluation of the Cobas TaqMan MTB test for the detection of Mycobacterium tuberculosis complex according to acid-fast-bacillus smear grades in respiratory specimens. J Clin Microbiol. 2015; 53:696–698.
28. Guglielmetti L, Mougari F, Lopes A, Raskine L, Cambau E. Human infections due to nontuberculous mycobacteria: the infectious diseases and clinical microbiology specialists' point of view. Future Microbiol. 2015; 10:1467–1483.
29. Somoskovi A, Salfinger M. Nontuberculous mycobacteria in respiratory infections: advances in diagnosis and identification. Clin Lab Med. 2014; 34:271–295.
30. Macheras E, Roux AL, Ripoll F, Sivadon-Tardy V, Gutierrez C, Gaillard JL, et al. Inaccuracy of single-target sequencing for discriminating species of the Mycobacterium abscessus group. J Clin Microbiol. 2009; 47:2596–2600.
31. Frothingham R, Wilson KH. Sequence-based differentiation of strains in the Mycobacterium avium complex. J Bacteriol. 1993; 175:2818–2825.
32. Ben Salah I, Adekambi T, Raoult D, Drancourt M. rpoB sequence-based identification of Mycobacterium avium complex species. Microbiology. 2008; 154(Pt 12):3715–3723.
33. Zelazny AM, Root JM, Shea YR, Colombo RE, Shamputa IC, Stock F, et al. Cohort study of molecular identification and typing of Mycobacterium abscessus, Mycobacterium massiliense, and Mycobacterium bolletii. J Clin Microbiol. 2009; 47:1985–1995.
34. Macheras E, Roux AL, Bastian S, Leao SC, Palaci M, Sivadon-Tardy V, et al. Multilocus sequence analysis and rpoB sequencing of Mycobacterium abscessus (sensu lato) strains. J Clin Microbiol. 2011; 49:491–499.
35. Jang MA, Koh WJ, Huh HJ, Kim SY, Jeon K, Ki CS, et al. Distribution of nontuberculous mycobacteria by multigene sequence-based typing and clinical significance of isolated strains. J Clin Microbiol. 2014; 52:1207–1212.
36. Koh WJ, Stout JE, Yew WW. Advances in the management of pulmonary disease due to Mycobacterium abscessus complex. Int J Tuberc Lung Dis. 2014; 18:1141–1148.
37. Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis. 2015; 21:1638–1646.
38. Cho YJ, Yi H, Chun J, Cho SN, Daley CL, Koh WJ, et al. The genome sequence of 'Mycobacterium massiliense' strain CIP 108297 suggests the independent taxonomic status of the Mycobacterium abscessus complex at the subspecies level. PLoS One. 2013; 8:e81560.
39. Sassi M, Drancourt M. Genome analysis reveals three genomospecies in Mycobacterium abscessus. BMC Genomics. 2014; 15:359.
40. Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009; 53:1367–1376.
41. Bastian S, Veziris N, Roux AL, Brossier F, Gaillard JL, Jarlier V, et al. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother. 2011; 55:775–781.
42. Brown-Elliott BA, Vasireddy S, Vasireddy R, Iakhiaeva E, Howard ST, Nash K, et al. Utility of sequencing the erm(41) gene in isolates of Mycobacterium abscessus subsp. abscessus with low and intermediate clarithromycin MICs. J Clin Microbiol. 2015; 53:1211–1215.
43. Choi GE, Shin SJ, Won CJ, Min KN, Oh T, Hahn MY, et al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med. 2012; 186:917–925.
44. Seng P, Rolain JM, Fournier PE, La Scola B, Drancourt M, Raoult D. MALDI-TOF-mass spectrometry applications in clinical microbiology. Future Microbiol. 2010; 5:1733–1754.
45. Buckwalter SP, Olson SL, Connelly BJ, Lucas BC, Rodning AA, Walchak RC, et al. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of Mycobacterium species, nocardia species, and other aerobic actinomycetes. J Clin Microbiol. 2016; 54:376–384.
46. Kodana M, Tarumoto N, Kawamura T, Saito T, Ohno H, Maesaki S, et al. Utility of the MALDI-TOF MS method to identify nontuberculous mycobacteria. J Infect Chemother. 2016; 22:32–35.
47. Mediavilla-Gradolph MC, De Toro-Peinado I, Bermudez-Ruiz MP, Garcia-Martinez Mde L, Ortega-Torres M, Montiel Quezel-Guerraz N, et al. Use of MALDI-TOF MS for identification of nontuberculous Mycobacterium species isolated from clinical specimens. Biomed Res Int. 2015; 2015:854078.
48. van Ingen J, Kuijper EJ. Drug susceptibility testing of nontuberculous mycobacteria. Future Microbiol. 2014; 9:1095–1110.
49. Griffith DE, Brown-Elliott BA, Langsjoen B, Zhang Y, Pan X, Girard W, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006; 174:928–934.
50. Clinical Laboratory Standards Institute. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes: approved standard. 2nd ed. Wayne: Clinical Laboratory Standards Institute;2011. CLSI No.M24-A2.
51. Czaja CA, Levin AR, Cox CW, Vargas D, Daley CL, Cott GR. Improvement in quality of life after therapy for Mycobacterium abscessus group lung infection: a prospective cohort study. Ann Am Thorac Soc. 2016; 13:40–48.
52. Egelund EF, Fennelly KP, Peloquin CA. Medications and monitoring in nontuberculous mycobacteria infections. Clin Chest Med. 2015; 36:55–66.
53. Wallace RJ Jr, Brown-Elliott BA, McNulty S, Philley JV, Killingley J, Wilson RW, et al. Macrolide/azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest. 2014; 146:276–282.
54. Jeong BH, Jeon K, Park HY, Kim SY, Lee KS, Huh HJ, et al. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015; 191:96–103.
55. Koh WJ, Hong G, Kim SY, Jeong BH, Park HY, Jeon K, et al. Treatment of refractory Mycobacterium avium complex lung disease with a moxifloxacin-containing regimen. Antimicrob Agents Chemother. 2013; 57:2281–2285.
56. Jo KW, Kim S, Lee JY, Lee SD, Kim WS, Kim DS, et al. Treatment outcomes of refractory MAC pulmonary disease treated with drugs with unclear efficacy. J Infect Chemother. 2014; 20:602–606.
57. Olivier KN, Shaw PA, Glaser TS, Bhattacharyya D, Fleshner M, Brewer CC, et al. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc. 2014; 11:30–35.
58. van Ingen J, Totten SE, Helstrom NK, Heifets LB, Boeree MJ, Daley CL. In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease. Antimicrob Agents Chemother. 2012; 56:6324–6327.
59. Ferro BE, Meletiadis J, Wattenberg M, de Jong A, van Soolingen D, Mouton JW, et al. Clofazimine prevents the regrowth of Mycobacterium abscessus and Mycobacterium avium type strains exposed to amikacin and clarithromycin. Antimicrob Agents Chemother. 2015; 60:1097–1105.
60. Jarand J, Davis JP, Cowie RL, Field SK, Fisher DA. Long term follow up of Mycobacterium avium complex lung disease in patients treated with regimens including clofazimine and/or rifampin. Chest. 2015; 10. 29. [Epub]. DOI: 10.1378/chest.15-0543.
61. Santin M, Dorca J, Alcaide F, Gonzalez L, Casas S, Lopez M, et al. Long-term relapses after 12-month treatment for Mycobacterium kansasii lung disease. Eur Respir J. 2009; 33:148–152.
62. Shitrit D, Baum GL, Priess R, Lavy A, Shitrit AB, Raz M, et al. Pulmonary Mycobacterium kansasii infection in Israel, 1999-2004: clinical features, drug susceptibility, and outcome. Chest. 2006; 129:771–776.
63. Moon SM, Park HY, Jeon K, Kim SY, Chung MJ, Huh HJ, et al. Clinical significance of Mycobacterium kansasii isolates from respiratory specimens. PLoS One. 2015; 10:e0139621.
64. Philley JV, Griffith DE. Treatment of slowly growing mycobacteria. Clin Chest Med. 2015; 36:79–90.
65. Kasperbauer SH, De Groote MA. The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015; 36:67–78.
66. Nie W, Duan H, Huang H, Lu Y, Bi D, Chu N. Species identification of Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii using rpoB and hsp65, and susceptibility testing to eight antibiotics. Int J Infect Dis. 2014; 25:170–174.
67. Kim SY, Kim CK, Bae IK, Jeong SH, Yim JJ, Jung JY, et al. The drug susceptibility profile and inducible resistance to macrolides of Mycobacterium abscessus and Mycobacterium massiliense in Korea. Diagn Microbiol Infect Dis. 2015; 81:107–111.
68. Lee SH, Yoo HK, Kim SH, Koh WJ, Kim CK, Park YK, et al. The drug resistance profile of Mycobacterium abscessus group strains from Korea. Ann Lab Med. 2014; 34:31–37.
69. Maurer FP, Bruderer VL, Ritter C, Castelberg C, Bloemberg GV, Bottger EC. Lack of antimicrobial bactericidal activity in Mycobacterium abscessus. Antimicrob Agents Chemother. 2014; 58:3828–3836.
70. Ferro BE, van Ingen J, Wattenberg M, van Soolingen D, Mouton JW. Time-kill kinetics of antibiotics active against rapidly growing mycobacteria. J Antimicrob Chemother. 2015; 70:811–817.
71. Maurer FP, Bruderer VL, Castelberg C, Ritter C, Scherbakov D, Bloemberg GV, et al. Aminoglycoside-modifying enzymes determine the innate susceptibility to aminoglycoside antibiotics in rapidly growing mycobacteria. J Antimicrob Chemother. 2015; 70:1412–1419.
72. Maurer FP, Castelberg C, Quiblier C, Bottger EC, Somoskovi A. Erm(41)-dependent inducible resistance to azithromycin and clarithromycin in clinical isolates of Mycobacterium abscessus. J Antimicrob Chemother. 2014; 69:1559–1563.
73. Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011; 183:405–410.
74. Kim HS, Lee KS, Koh WJ, Jeon K, Lee EJ, Kang H, et al. Serial CT findings of Mycobacterium massiliense pulmonary disease compared with Mycobacterium abscessus disease after treatment with antibiotic therapy. Radiology. 2012; 263:260–270.
75. Lyu J, Kim BJ, Kim BJ, Song JW, Choi CM, Oh YM, et al. A shorter treatment duration may be sufficient for patients with Mycobacterium massiliense lung disease than with Mycobacterium abscessus lung disease. Respir Med. 2014; 108:1706–1712.
76. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011; 52:565–571.
77. Mirsaeidi M, Farshidpour M, Ebrahimi G, Aliberti S, Falkinham JO 3rd. Management of nontuberculous mycobacterial infection in the elderly. Eur J Intern Med. 2014; 25:356–363.
78. van Ingen J, Egelund EF, Levin A, Totten SE, Boeree MJ, Mouton JW, et al. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med. 2012; 186:559–565.
79. van Ingen J, Ferro BE, Hoefsloot W, Boeree MJ, van Soolingen D. Drug treatment of pulmonary nontuberculous mycobacterial disease in HIV-negative patients: the evidence. Expert Rev Anti Infect Ther. 2013; 11:1065–1077.
80. Westphal JF. Macrolide-induced clinically relevant drug interactions with cytochrome P-450A (CYP) 3A4: an update focused on clarithromycin, azithromycin and dirithromycin. Br J Clin Pharmacol. 2000; 50:285–295.
81. Rose SJ, Neville ME, Gupta R, Bermudez LE. Delivery of aerosolized liposomal amikacin as a novel approach for the treatment of nontuberculous mycobacteria in an experimental model of pulmonary infection. PLoS One. 2014; 9:e108703.
82. Winthrop KL, Ku JH, Marras TK, Griffith DE, Daley CL, Olivier KN, et al. The tolerability of linezolid in the treatment of nontuberculous mycobacterial disease. Eur Respir J. 2015; 45:1177–1179.
83. Wallace RJ Jr, Brown-Elliott BA, Crist CJ, Mann L, Wilson RW. Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrob Agents Chemother. 2002; 46:3164–3167.
84. Wallace RJ Jr, Dukart G, Brown-Elliott BA, Griffith DE, Scerpella EG, Marshall B. Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J Antimicrob Chemother. 2014; 69:1945–1953.
85. Huang CW, Chen JH, Hu ST, Huang WC, Lee YC, Huang CC, et al. Synergistic activities of tigecycline with clarithromycin or amikacin against rapidly growing mycobacteria in Taiwan. Int J Antimicrob Agents. 2013; 41:218–223.
86. Tang S, Yao L, Hao X, Liu Y, Zeng L, Liu G, et al. Clofazimine for the treatment of multidrug-resistant tuberculosis: prospective, multicenter, randomized controlled study in China. Clin Infect Dis. 2015; 60:1361–1367.
87. Shen GH, Wu BD, Hu ST, Lin CF, Wu KM, Chen JH. High efficacy of clofazimine and its synergistic effect with amikacin against rapidly growing mycobacteria. Int J Antimicrob Agents. 2010; 35:400–404.
88. Philley JV, Wallace RJ Jr, Benwill JL, Taskar V, Brown-Elliott BA, Thakkar F, et al. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest. 2015; 148:499–506.
89. Bjarnsholt T, Hoiby N, Donelli G, Imbert C, Forsberg A. Understanding biofilms: are we there yet? FEMS Immunol Med Microbiol. 2012; 65:125–126.
90. Qvist T, Eickhardt S, Kragh KN, Andersen CB, Iversen M, Hoiby N, et al. Chronic pulmonary disease with Mycobacterium abscessus complex is a biofilm infection. Eur Respir J. 2015; 46:1823–1826.
91. Fennelly KP, Ojano-Dirain C, Yang Q, Liu L, Lu L, Progulske-Fox A, et al. Biofilm formation by Mycobacterium abscessus in a lung cavity. Am J Respir Crit Care Med. 2016; 193:692–693.
92. Rose SJ, Babrak LM, Bermudez LE. Mycobacterium avium possesses extracellular DNA that contributes to biofilm formation, structural integrity, and tolerance to antibiotics. PLoS One. 2015; 10:e0128772.
93. Aung TT, Yam JK, Lin S, Salleh SM, Givskov M, Liu S, et al. Biofilms of pathogenic nontuberculous mycobacteria targeted by new therapeutic approaches. Antimicrob Agents Chemother. 2015; 60:24–35.
94. Griffith DE, Aksamit TR. Therapy of refractory nontuberculous mycobacterial lung disease. Curr Opin Infect Dis. 2012; 25:218–227.
95. Mitchell JD. Surgical approach to pulmonary nontuberculous mycobacterial infections. Clin Chest Med. 2015; 36:117–122.
96. Yu JA, Pomerantz M, Bishop A, Weyant MJ, Mitchell JD. Lady Windermere revisited: treatment with thoracoscopic lobectomy/segmentectomy for right middle lobe and lingular bronchiectasis associated with non-tuberculous mycobacterial disease. Eur J Cardiothorac Surg. 2011; 40:671–675.
97. Shiraishi Y, Katsuragi N, Kita H, Hyogotani A, Saito MH, Shimoda K. Adjuvant surgical treatment of nontuberculous mycobacterial lung disease. Ann Thorac Surg. 2013; 96:287–291.
98. Kang HK, Park HY, Kim D, Jeong BH, Jeon K, Cho JH, et al. Treatment outcomes of adjuvant resectional surgery for nontuberculous mycobacterial lung disease. BMC Infect Dis. 2015; 15:76.
99. Shiraishi Y. Surgical treatment of nontuberculous mycobacterial lung disease. Gen Thorac Cardiovasc Surg. 2014; 62:475–480.
100. Shiraishi Y. Current status of nontuberculous mycobacterial surgery in Japan: analysis of data from the annual survey by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2016; 64:14–17.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download