Abstract
Objective
The aim of this experimental study was to evaluate the effects of direct electrical current stimulation (DECS) on bone regeneration in response to an expansion of the inter-premaxillary suture in the rat.
Methods
Sixteen 50 - 60 days old Wistar male rats were separated into two equal groups (control and experimental). Both groups were subjected to expansion, and 30-gram of force was applied to the maxillary incisors with helical-spring. In the experimental group, two metallic-screws were placed at lateral parts of the maxillary segments. Electrodes were connected to the screws. The device was activated with current adjustment to measure 10 µA continuously and the current was monitored daily during the expansion and early-retention phase. Bone regeneration in the sutural area was histomorphometrically evaluated including new-bone area (µm2), bone perimeter (µm), feret's diameter (µm) and newly formed bone (%) parameters. Kruskal-Wallis rank and Mann-Whitney U tests were used for statistical evaluation at p < 0.05 level.
Results
Statistical analysis showed significant differences between groups for all investigated histomorphometric parameters. New bone area (p = 0.002), bone perimeter (p = 0.004), feret's diameter (p = 0.002) and newly formed bone percentage (p = 0.002) measurements were significantly higher in the experimental group than the control group. Bone histomorphometric measurements revealed that bone architecture in the DECS group was improved.
Rapid palatal expansion (RPE) is the main treatment alternative to correct narrow dental arch and skeletal base in routine clinical orthodontic practice. During RPE the mid-palatal suture is widened and the two maxillae are forced apart by an appliance anchored to the buccal teeth. Mechanical stimulation by RPE in the expanding suture induces biological responses of skeletal regeneration that is accomplished by a flow of biological processes that may include differentiation of pluripotential tissue, angiogenesis, mineralization, and remodeling.1 There are complex interactions between bone-forming osteoblasts and other cells present within the suture microenvironment, particularly vascular endothelial cells that may be pivotal members of a complex interactive communication network in bone.1
The regulation of bone metabolism and stresses generated on mid-palatal and circum-maxillary sutures depend on many factors. It is well-known that even after a period of retention the expanded maxillae can 'rebound' to their original positions, in some cases by as much as 90 percent.2-4 Haas5 concluded that complete ossification of the suture margins lasts 60 to 90 days. Although the reason for the post-expansion relapse is not fully understood, the quality and rapidity of bone deposited in the mid-palatal suture during and after expansion may influence the relapse.6 It could be postulated that accelerated bone formation in the suture after expansion may reduce the amount of time required for retention and prevent the maxillae from relapsing.6-10
Interest in methods of accelerating bone healing persists in many surgical orthopedical concepts. The promise of successful invasive and noninvasive alternatives to accelerate bone healing continues to be realized.11 In a number of in vivo and in vitro experiments with embryonic bone, cartilage cells, and mature bone, cells respond to electromagnetic stimulation by increased proliferation, matrix production or changes in calcium flux.11-16 The induction of electric current in bone not only prevents the bone loss of functional disuse, but also induces new bone formation.17 Direct electrical current stimulation (DECS) has already been applied clinically to treat delayed fracture union and pseudoarthrosis.14 DECS is used clinically to treat different orthopedic problems. It provided a significant increase in new-bone formation, and a higher mechanical strength of healing.17
Animal studies demonstrated that DECS increases the healing rates of bones also in the maxillofacial region. Hagiwara and Bell18 investigated the effect of DECS on distraction osteogenesis of the rabbit's mandible. They applied DECS (10 µA) during the distraction phase. Image analysis in areas of newly formed bone showed that there was a greater amount of new bone formation in the stimulation group than in the control, 10 and 20 days after distraction. El-Hakim et al.17 carried out research to test the effect of DECS on distraction osteogenesis in goats histomorphometrically and suggested that DECS display positive results on mandibular distraction when this stimulation is applied to the distraction zone during activation or consolidation periods.
The goal of combining electrical stimulation with a RPE procedure is to accelerate the process of regeneration of bone in an expanding suture so that the retention devices can be removed earlier. The aim of this experimental study was to evaluate the effects of DECS on bone formation in the expanded inter-premaxillary suture in the rat.
Sixteen 50 - 60 days old Wistar male rats with a mean weight of 196.19 ± 20.35 grams were used. All animals were housed in polycarbonate cages, subjected to a 12-hour light - dark cycle at the constant temperature of 23℃ and fed a standard pellet diet (Expanded pellets, Stepfield Witham, Essex, UK) with tap water ad libitum. Permission was obtained from the Gulhane Military Medical Academy; Ethics Committee of Experimental Animals after the Research Scientific Committee at the same institution had approved the experimental protocol. The experiments were carried out in the Department of Experimental Animals, Research and Development Center, Gulhane Military Medical Academy. Animals were randomly separated into two groups (control and experimental).
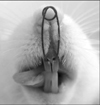
The animals were anaesthetised with an intramuscular injection of Xylasine (Bayer, Istanbul, Turkey) and Ketamine (Parke-Davis, Istanbul, Turkey) at 0.5 ml/kg and 1 ml/kg body weight, respectively. The expansion appliances were helical springs fabricated from 0.014 inch stainless steel wire inserted in holes drilled close to the gingival margins of both upper incisors (Fig 1). The springs were activated to deliver a force of 30 grams and were not reactivated during the five-day expansion period.
After five days the springs were removed and replaced with short lengths of rectangular retaining wire. The retaining wire maintained the separation during the retention phase. The distance between the mesial edges of the maxillary incisors was measured at the beginning and on the fifth day of the expansion with a digital-caliper (MSI-Viking Gage, Duncan, SC, USA). Occlusal radiographs were taken at three stages: at the baseline, end of expansion and at the end of the retention periods.
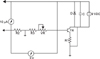
The electrical generator used to stimulate the suture area has been manufactured and calibrated by the Department of Biomedical Engineering, Gulhane Military Medical Academy. In the experimental group of 8 rats, direct current electrical stimulators (Figs 2 and 3) consisting of field effect transistors and resistors in circuit with a 9-Volt battery were placed on the back of the rats 24 hours after the expansion process. Two titanium screws (3 mm length and 1.5 mm diameter) were placed at the most lateral parts of the right and left maxillary segments by local anesthesia. The electrodes were connected to the screws. The device was activated with current adjustment to measure 3 volt and 10 µA continuously and the current was monitored using voltmeter and ampermeter daily during the expansion (2nd to 5th days of expansion) and early-retention phase (2nd to 7th days of retention). In study animals, the device cathode was connected to the right screw and the anode was connected to the left screw.
After the experimental procedure (5 days expansion and 7 days retention period), the rats were sacrificed with an overdose of Ketamine and Xylasine and their premaxillae were dissected out and fixed in 10 per cent formalin. After fixation the retaining wires were removed and the premaxillae were decalcified with 5 per cent formic acid for three days. After decalcification the premaxillae were cut into blocks with one cut passing through the incisor crowns at the alveolar crest and perpendicular to the sagittal plane, and the second cut located 4 mm apical to the first cut. The sections were rinsed, trimmed and embedded in paraffin. The paraffin blocks were sectioned serially at 5 µm intervals.
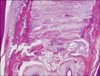
Histological sections were stained with hematoxylin and eosin prior to optical microscope examination (Fig 4). Bone histomorphometric measurement was performed 200 µm beneath the oral surface of the osseous palate because bone formation of the surface area was sometimes irregular and not suitable for quantitative measurement. Measurements were based on observations of the sections under a microscope and calculated using an image analysis program. For this purpose a microscope and digital camera system (Olympus CX41/DP25 Research System, Olympus, Tokyo, Japan) were used.
Biometric analysis for the amount of expansion was done by image analysis software at the most anterior part of the pre-maxilla on histological sections.
Bone regeneration in the sutural area was evaluated by the histomorphometric method and new-bone area (µm2), bone perimeter (µm), feret's diameter (µm) (the longest distance between any two points along the selection boundary, also known as maximum calliper) and newly formed bone (%) parameters were evaluated.
Measurements were performed double-blinded by two of the contributors, with the final results being an average of values from these two separate evaluations. Two histological sections were analyzed for each animal. The relevant areas on the slides were predefined and two representative digital images were captured under × 400 magnification. Computer-assisted image-analysis software, Image-J,19 was used to make measurements histomorphometrically. For this purpose, two separate image analysis macro was prepared by one of the authors (Y.K.). In the first macro, images were enhanced to increase the contrast between bone and surrounding tissues. The user needed to draw the outline of the newly formed islands of bone. The program allowed the user to outline more than one bone islands (Fig 5). At the end of this macro, results window appeared showing some basic planimetric measurements of the outlined objects (Fig 6).
With the aid of the second macro, the obtained images were opened, enhanced in the same manner, and a grid system consisting of squares with areas of 1,000 µm2 each was superimposed. Then, the user is required to click on the intersections of the grid only over newly formed bone. After labeling newly formed bone, the user was guided to select and mark the non-ossified areas in same manner. At the end of the macro, the program calculated percentages of newly formed bone and gave results (Fig 7).
Statistics were analyzed with the Statistical Package for Social Sciences 13.0 (SPSS for Windows, SPSS Inc, Chicago, IL, USA). A non-parametric test, the Kruskal-Wallis one-way analysis of variance was used to compare the groups, and a one-sided Mann-Whitney U test was used to determine which groups were significantly different. Probability values less than 0.05 were accepted as significant.
All animals survived to the end of the study. Deep mucosal infection, dehiscences or other adverse effects were not observed in any animals. The expansion appliance was well tolerated and the animals gained weight. The body weight of two rats in the experimental group decreased during the expansion period and one rat in the control group decreased during the retention period, but subsequently recovered. As no statistically significant changes in body weight were found during the expansion and retention periods there was no reason to weight-correct the data.
Suture width measurements from histological sections showed that the inter-premaxillary suture was expanded following application of an activated helical loop. The results indicated that the mean amount of expansion was less in the DECS group (323.39 ± 23.48 µm) than the control (331.61 ± 24.80 µm). However, the statistical analysis showed no significant differences (p = 0.462) (Table 1).
No obvious morphological differences were noted between the cathode side and the anode side.
Statistical analysis showed significant differences between the two groups for all investigated histomorphometric parameters. New-bone area (p = 0.002), bone perimeter (p = 0.004), Feret's diameter (p = 0.002), and newly formed bone (p = 0.002) measurements showed statistically significant differences (Table 2). For all histomorphometric parameters, DECS group showed more positive results related to the new bone formation, revealing that bone architecture in the treatment group was improved.
To our knowledge, this study is the first to report that application of electrical stimulation increased bone healing in the expanded inter-premaxillary suture in the rat. More new bone was deposited in the expanded suture following electrical current application than in the control suture, leading to a more advanced stage of healing.
Orthopedic surgeons have carried out much research that have shown applications of different mechanical stimulations or several pharmacological agents for increasing bone formation. In the orthodontic literature few researches have been carried out to stimulate regeneration in the mid-palatal/inter-premaxillary suture after expansion. Saito and Shimizu6 evaluated the low-power laser irradiation and Sawada and Shimizu7 applied a single dose of Transforming Growth Factor-β1 for stimulation of expanding suture in the rat. In both studies significantly stimulated bone regeneration in the mid-palatal suture was found. Recently, we investigated the effects of dietary boron in rabbits and locally administered ED-71 and vitamin E on bone formation in the mid-palatal suture in rats, and found that these agents stimulated bone regeneration during the expansion and retention periods.8-10
El-Hakim et al.17 and Hagiwara and Bell18 reported that electrical stimulation during gradual distraction promotes new bone formation during activation or consolidation periods. Thus, in the current study, the effect of DECS on bone regeneration in response to expansion of the inter-premaxillary suture was investigated in rats and demonstrated an increase of newly formed mineralized bone area in the suture.
Before the experimental period, the present authors carried out a pilot study on 4 rats (2 only-expansion and 2 sham-operated rats) in order to evaluate the pure effect of the surgical procedure (metallic screw placement) on the animals' health and on bone healing; and at the end of experimental period (12 days = 5 days expansion and 7 days retention) compared the shamoperated group with the only-expansion group. After the experimental procedure and histomorphometric evaluation, no difference was observed between the two groups' bone healing and measured histomorphometric values. The animals tolerated the appliance and the implanted metallic screws well. It was thought that similar healing of the two groups was due to the localization of the screws. Metallic screws were placed at the most lateral parts of the right and left maxillary segments by local anesthesia which gave minor trauma and did not affect the healing procedure. The wound from the implantation of the screw was adequately healed by the day following surgery. After operation oral irrigation was performed in all animals. Mucosal infection, dehiscence or other adverse effects were not observed in any of the animals. In the pilot study, no weight loss was observed in both groups during the experimental period.
Various animal models have been described in the literature to investigate the process of bone healing. Swennen et al.20 reported that different animals such as dogs, rabbits, sheep, minipig, monkeys, rats and cats were used as an animal model to study the effect of electric currents on bone. While monkey and cat have similar maxillary sutures in most aspects to that of man and have been used in maxillary expansion studies, the ideal animals with which to obtain a clear picture of bone and sutural changes under stimulation are rabbits and rats.21 In this study, rats were used as an experimental animal model. Results of this study proved that rats are considered a suitable experimental animal model to study the effect of electricity on bone healing in the sutural area. The primate animal may be the ideal model for bone healing experimentation because of anatomical and wound healing similarities to the human facial skeleton.18 Therefore; additional clinical studies are indicated to confirm the effect of electrical stimulation on maxillary expansion in primate models.
The thickness of the normal inter-premaxillary suture in young rats approximated 20 - 60 µm.22 Burstone and Shafer22 determined that expansion of the suture over a period of five days 'opened' the suture, on average 377 ± 104 µm. In the current study, the inter-premaxillary suture was opened by helical springs that were applied buccally, and occlusal radiographs showed wide separation of inter-premaxillary bones after 5 days of expansion. The sutural width, measured at the most anterior part of the pre-maxilla at 200 µm under the surface of the osseous palate facing the oral cavity were found to range between 299.21 µm and 386.21 µm. The amount of expansion determined by image analysis software in all groups showed no statistically significant differences.
This is in agreement with El-Hakim et al.17 and Hagiwara and Bell18 who reported that electrical stimulation during gradual distraction promotes new bone formation during activation or consolidation periods.
Gradual traction can be applied not only to long bones but also to the maxillofacial area, usually to form new bone.23 In most studies of distraction osteogenesis for long bones or mandible lengthening, the authors have reported new bone formation by intramembranous ossification.23 In the present study, it was seen that there was no cartilaginous or fibrocartilaginous tissue in newly formed bone, and the ossification was defined as intramembranous.
The mechanisms by which such stimulation could interact with biological systems to accelerate healing have not been explained yet. One potential mechanism could involve stimulation of macrophage migration to the site of the wound.17 Electric fields cause macrophage migration on laminin or fibronectin coated substrates without inducing podosome formation or changes in cellular morphology.17 The produced electricity might stimulate the undifferentiated mesenchymal cells in the bone marrow to proliferate and transform into osteoblasts.24 Current findings indicate that electrical stimulation may enhance bone formation on maxillary expansion when this stimulation is simultaneously applied to the expanding inter-premaxillary area during the expansion and early-retention period. The mechanism of the positive results may be caused by promoting both, the differentiation of mesenchymal cells into osteoblasts and the proliferation of osteoblasts in the expansion zone.
Additionally, Block and Brister13 reported that enlargement of calcium gates during electric stimulation occurred and this resulted in more incorporation of calcium into the cells. Blank12 described how calcium was incorporated in cells by enlargement of the calcium gates during electrical stimulation. Cane et al.15 suggested that an electric field might accelerate the healing process through enhancement of the enzymatic activity of the repaired tissue.
Figures and Tables
Fig. 5
Histomorphometric measurements of newly formed bone area (µm2). Image enhancement and outlining of the area of interest, which is newly formed bone.

Fig. 6
At the end of the first macro, results window shows the basic planimetric measurements calculated from the outlined objects.

Fig. 7
Histomorphometric measurements of newly formed bone (percentage). The number of grid intersections on new bone and non-osseous connective tissue were counted and the percentage of new bone calculated.

ACKNOWLEDGMENTS
The authors thank Gulhane Military Medical Academy, Department of Biomedical Engineering, for their support to manufacture and calibrate the electrical generator that was used in this project.
References
1. Choi IH, Chung CY, Cho TJ, Yoo WJ. Angiogenesis and mineralization during distraction osteogenesis. J Korean Med Sci. 2002. 17:435–447.

2. Krebs AA. Midpalatal suture expansion studied by the implant method over a seven-year period. Trans Eur Orthod Soc. 1964. 40:131–142.
3. Vardimon AD, Graber TM, Voss LR. Stability of magnetic versus mechanical palatal expansion. Eur J Orthod. 1989. 11:107–115.

4. Timms DJ. Long term follow-up of cases treated by rapid maxillary expansion. Trans Eur Orthod Soc. 1976. 52:211–215.
5. Haas AJ. The treatment of maxillary deficiency by opening the midpalatal suture. Angle Orthod. 1965. 35:200–217.
6. Saito S, Shimizu N. Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop. 1997. 111:525–532.

7. Sawada M, Shimizu N. Stimulation of bone formation in the expanding mid-palatal suture by transforming growth factor-beta 1 in the rat. Eur J Orthod. 1996. 18:169–179.

8. Uysal T, Ustdal A, Sonmez MF, Ozturk F. Stimulation of bone formation by dietary boron in an orthopedically expanded suture in rabbits. Angle Orthod. 2009. 79:984–990.

9. Uysal T, Amasyali M, Enhos S, Sonmez MF, Sagdic D. Effect of ED-71, a new active vitamin D analog, on bone formation in an orthopedically expanded suture in rats. A histomorphometric study. Eur J Dent. 2009. 3:165–172.

10. Uysal T, Amasyali M, Olmez H, Karslioglu Y, Gunhan O. Stimulation of bone formation in the expanding inter-premaxillary suture by vitamin E, in rat. Korean J Orthod. 2009. 39:337–347.

11. Albert SF, Wong E. Electrical stimulation of bone repair. Clin Podiatr Med Surg. 1991. 8:923–935.
12. Blank M. Blank M, Findl E, editors. The influence of surface charge on oligomeric reactions as a basis for channel dynamics. Mechanistic Approaches to interactions of electric and electromagnetic fields with living systems. 1987. New York: Plenum Press;151–160.

13. Block MS, Brister GD. Use of distraction osteogenesis for maxillary advancement: preliminary results. J Oral Maxillofac Surg. 1994. 52:282–286.

14. Brighton CT, Black J, Friedenberg ZB, Esterhai JL, Day LJ, Connolly JF. A multicenter study of the treatment of non-union with constant direct current. J Bone Joint Surg Am. 1981. 63:2–13.

15. Cane V, Zaffe D, Cavani F, Botti P, Soana S. PEMFs modulate the enzymatic activity during the bone repair process. Bone. 1996. 19:133S.

16. Kim DH, Park YG, Kang SG. The effects of electrical current from a micro-electrical device on tooth movement. Korean J Orthod. 2008. 38:337–346.

17. El-Hakim IE, Azim AM, El-Hassan MF, Maree SM. Preliminary investigation into the effects of electrical stimulation on mandibular distraction osteogenesis in goats. Int J Oral Maxillofac Surg. 2004. 33:42–47.

18. Hagiwara T, Bell WH. Effect of electrical stimulation on mandibular distraction osteogenesis. J Craniomaxillofac Surg. 2000. 28:12–19.

19. Rasband WS. Image-J. 1997-2008. Bethesda, Maryland, USA: U.S. National Institutes of Health;
http://rsb.info.nih.gov/ij/.
20. Swennen G, Dempf R, Schliephage H. Cranio-facial distraction osteogenesis: a review of the literature. Part II: experimental studies. Int J Oral Maxillofac Surg. 2002. 31:123–135.

22. Burstone CJ, Shafer WG. Sutural expansion by controlled mechanical stress in the rat. J Dent Res. 1959. 38:534–540.

23. Cope JB, Samchukov ML. Mineralization dynamics of regenerate bone during mandibular osteodistraction. Int J Oral Maxillofac Surg. 2001. 30:234–242.

24. Matsunaga S. Histological, histochemical investigations of constant direct current stimulated intramedullary callus. Nippon Seikeigeka Gakkai Zasshi. 1986. 60:1293–1303.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download