Abstract
Purpose
Materials and Methods
Results
ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTIONS:
Data curation: Hye Won Chung, Jin Ju Kim, and Jong-Baeck Lim.
Formal analysis: Hye Won Chung, Jin Ju Kim, Jae Il Choi, and Hae Rim Lee.
Funding acquisition: Jong-Baeck Lim.
Investigation: Jong-Baeck Lim.
Methodology: Jae Il Choi and Hae Rim Lee.
Project administration: Hye Won Chung, Jin Ju Kim, Jae Il Choi, and Jong-Baeck Lim.
Resources: Jong-Baeck Lim.
Software: Hye Won Chung and Jae Il Choi.
Supervision: Jong-Baeck Lim.
Validation: Hye Won Chung and Jong-Baeck Lim.
Visualization: Hye Won Chung and Jong-Baeck Lim.
Writing—original draft: Hye Won Chung and Jong-Baeck Lim.
Writing—review & editing: Hye Won Chung and Jong-Baeck Lim.
References
Fig. 1
ROC curves and AUCs of blood ADAM 8 and CEA for prediction of gastric cancer in independent validation dataset. ADAM 8, a disintegrin and metalloproteinase domain 8; CEA, carcinoembryonic antigen; AUC, area under curve; ROC, receiver operating characteristic curve.
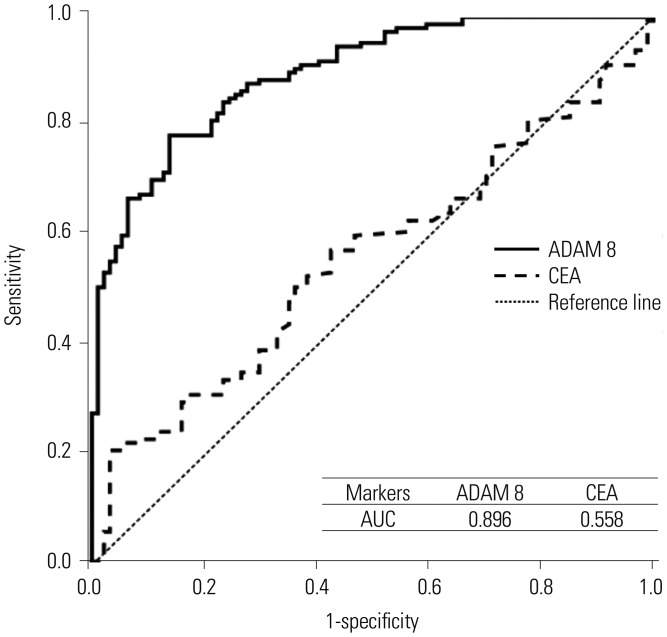
Table 1
Serum Levels of ADAM 8 and CEA according to GC Carcinogenic Sequence (Upper Panel) and between Non-Cancer and Cancer Groups (Lower Panel) in Initial Training Dataset
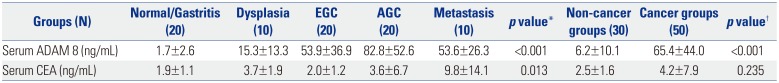
ADAM 8, a disintegrin and metalloproteinase domain 8; CEA, carcinoembryonic antigen; GC, gastric cancer; EGC, early gastric cancer; AGC, advanced gastric cancer. Values are expressed as mean±standard deviation.
*One-way analysis of variance test with multiple comparisons using post-hoc Bonferroni method was used to compare differences in means among disease groups; †An independent sample t-test was used to compare differences in means between non-cancer and cancer groups. p<0.05 (two-tailed) was considered statistically significant.
Table 2
Serum Levels of ADAM 8 and CEA according to GC Carcinogenic Sequence (Upper Panel) and between Non-Cancer and Cancer Groups (Lower Panel) in Validation Dataset
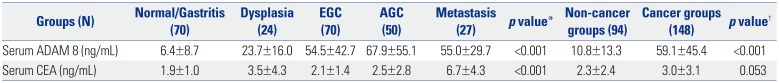
ADAM 8, a disintegrin and metalloproteinase domain 8; CEA, carcinoembryonic antigen; GC, gastric cancer; EGC, early gastric cancer; AGC, advanced gastric cancer. Values are expressed as mean±standard deviation.
*One-way analysis of variance test with multiple comparisons using post-hoc Bonferroni method was used to compare differences in means among disease groups; †An independent sample t-test was used to compare differences in means between non-cancer and cancer groups. p<0.05 (two-tailed) was considered statistically significant.
Table 3
Relationships between Serum ADAM 8 and Clinicopathological Characteristics of GC in Validation Dataset
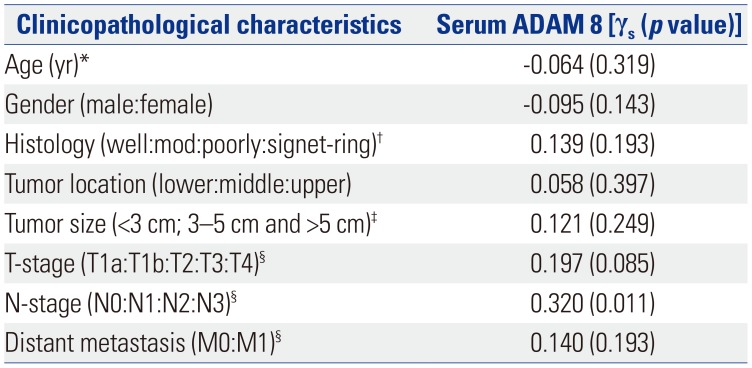
ADAM 8, a disintegrin and metalloproteinase domain 8; GC, gastric cancer.
‘γs’ means ‘Spearman's correlation coefficient. p<0.05 (two-tailed) was considered statistically significant’.
*This was a continuous variable. Therefore, correlation was evaluated by Pearson's correlation (γp); †‘Well:mod:poorly:signet-ring’ means ‘well-differentiated carcinoma:moderate-differentiated carcinoma:poorly-differentiated carcinoma:signet-ring cell carcinoma’; ‡Tumor size was classified into three groups: <3 cm, 3–5 cm, and >5 cm; §TNM stage was evaluated according to the 7th International Union Against Cancer-TNM stage guidelines.
Table 4
Comparison of Sensitivity and Specificity of Serum ADAM 8, CEA and Their Combination as Single Markers and/or as a Multiple-Markers Panel for Prediction of GC Determined by Logistic Regression Analysis in Validation Dataset
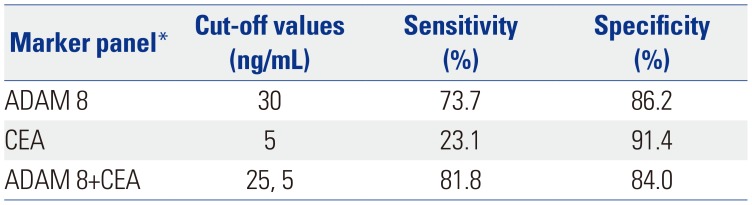
| Marker panel* | Cut-off values | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| ADAM 8 | 30 | 73.7 | 86.2 |
| CEA | 5 | 23.1 | 91.4 |
| ADAM 8+CEA | 25, 5 | 81.8 | 84.0 |
Table 5
Pearson's Correlations among Serum Levels of IL-23, SDF-1α, IL-8, sCD40L, and ADAM 8 in Validation Dataset

| IL-23 | SDF-1α | IL-8 | sCD40L | |
|---|---|---|---|---|
| ADAM 8 | 0.235 (0.036)* | −0.233 (0.037)* | 0.113 (0.313) | 0.043 (0.702) |
ADAM 8, a disintegrin and metalloproteinase domain 8; IL-23, interleukin-23; SDF-1α, stromal cell-derived factor 1α; IL-8, interleukin-8; sCD40L, soluble CD40 ligand.
Values are presented as γp (p value). ‘γp’ means ‘Pearson's correlation coefficient’. p<0.05 (two-tailed) was considered statistically significant.
*Statistically significant values.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download