Abstract
Purpose
Foxo3 in female reproduction has been reported to regulate proliferation of granulose cells that form follicles. There are no reports so far that discuss on the role of Foxo3 in males. This study was designed to outline the role of Foxo3 in the testes.
Materials and Methods
Testes from mice at birth to postpartum week (PPW) 5 were isolated and examined for the expression of Foxo3 using immunostaining. To elucidate role of Foxo3 in Leydig cells, R2C cells were treated with luteinizing hormone (LH) and the phosphorylation of Foxo3. Testosterone and steroidogenic acute regulatory (StAR) protein levels were measured after constitutive active [triple mutant (TM)] human FOXO3 adenovirus was transduced and StAR promoter assay was performed.
Results
Foxo3 expression in the testicles started from birth and lasted until PPW 3. After PPW 3, most Foxo3 expression occurred in the nuclei of Leydig cells; however, at PPW 5, Foxo3 was expressed in both the nucleus and cytoplasm. When R2C cells were treated with luteinizing hormone, Foxo3 phosphorylation levels by AKT increased. After blocking the PI3K pathway, LH-induced phosphorylated Foxo3 levels decreased, indicating that LH signaling regulates Foxo3 localization. When active FOXO3-TM adenovirus was introduced into a Leydig tumor cell line, the concentrations of testosterone and StAR protein decreased. When FOXO3 and a StAR promoter vector were co-transfected into HEK293 cells for a reporter assay, FOXO3 inhibited the StAR promoter.
The main functions of the testes are testosterone production and spermatogenesis. These two functions are controlled by the hypothalamus-pituitary-gonad axis. Gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the production of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in the anterior pituitary.1 FSH binds to its receptor on the surface of Sertoli cells to regulate spermatogenesis.23 LH binds to its receptor on the Leydig cell membrane to stimulate testosterone production. The testosterone produced by LH negatively regulates GnRH production in the hypothalamus.456
There are four types of forkhead box class O (Foxo) transcription factors: Foxo1 (FKHR, forkhead in rhabdomyosarcoma), Foxo3 (FKHRL2, FKHR-like1), Foxo4 (AFX, acute-lymphocytic-leukemia-1), and Foxo6. These Foxo proteins regulate stress responses, aging, insulin sensitivity, and ontogenesis,78 and their transcription is inhibited by phosphoinositide 3-kinase (PI3K). PI3K signaling phosphorylates AKT, which then phosphorylates Foxo3 at Ser24, Thr32, and Ser56 residues. These phosphorylated sites recruit 14-3-3 protein to guide Foxo3 from the nucleus into the cytoplasm. Finally, Foxo3 is removed by proteasomes.9
In female reproduction, Foxo1, Foxo3, and Foxo4 are expressed in the granulosa cells at various stages of follicle development.10 Foxo1 in granulosa cells inhibits cyclin D2 gene expression and increases the nuclear localization of p27kip proteins, which makes it a key regulator of G1/S transition. Foxo proteins also play an important role in regulating ovarian function by pituitary gonadotropins.1011 In a previous study, Foxo3-null female mice exhibited age-dependent fertility issues and were completely sterile at 10 weeks or older. In Foxo3-/- ovaries at 9.5 weeks, oocytes in developing follicles appeared to have degenerated, reflecting atretic change. At 12 weeks, Foxo3-/- ovaries had no developing follicles. These indicated that Foxo3 is important in ovarian follicular development.111213 Meanwhile, in males, germ line specific Foxo1 KO mice showed defective proliferative expansion and small testes, which was not due to cell death, but rather to renewal of spermatogonial stem cells.14 However, the function of Foxo3 in Leydig cells is not clear.15
Foxo3 is important not only in females, but also in males. Foxo3 expression and location are likely to be dynamic throughout life. In this study, Foxo3 expression and location were investigated from mouse embryonic stage to 12 weeks, and the role of Foxo3 in Leydig cells was investigated to outline the function and regulation of Leydig cells.
C57/BL6 (Jackson Labs, CA, USA) male mice were housed in a barrier facility under normal light and dark conditions and fed ad libitum. Testes were isolated at postpartum days (PPD) 1 and 5 and postpartum weeks (PPW) 3, 4, 5, and 12. Testes were removed, fixed in 10% formalin, and embedded in paraffin. All procedures were approved by the Animal Care and Use Committees at Yonsei University College of Medicine and Northwestern University.
The mStARp-Luc plasmid was constructed by inserting the mouse steroidogenic acute regulatory (StAR) promoter (2730 bps) into the multiple cloning site of pGL3 basic vector. As synthetic poly A (spa) region in PGL3 basic vector contained two FOXO binding sequences, we removed them using NotI and KpnI.16 The human FOXO3 triple mutant (FOXO3-TM) was generated by substituting Thr32, Ser253, and Ser315 with alanine residues. This FOXO3-TM cannot be phosphorylated by Akt and is constitutively activated.1718 Recombinant adenoviral vectors carrying FOXO3 [wild type (WT) or TM] were generated as previously described.8 Adenovirus carrying β-galactosidase (Ad-Gal) was used as a control.8
MA10 and R2C cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 15% horse serum (HS), 5% fetal bovine serum (FBS), and 100 U/mL penicillin and streptomycin. The cultures were maintained at 37℃ in a humidified atmosphere of 5% CO2. R2C cells were cultured in DMEM with 15% FBS and 5% HS, and infected with β-gal adenovirus (5 pfu) or TM FOXO3 virus (1 or 5 pfu) for 24 hr. The cultures were then maintained in serum free media for 48 hr. Testosterone levels in the medium were measured by radioimmunoassay (Diagnostic Systems Laboratories Inc., Webster, TX, USA), and cells were collected for a Western blot. R2C cells were plated and maintained in serum-free medium for 24 hr before treatment with 10 IU IVF-C (human chromic gonadotropin, LG Life Science, Seoul, Korea) or vehicle. To block the PI3K pathway, 20 µM of LY293002 were applied for 2 hr and then 10 IU of IVF-C were added.
One day before transfection, 293FT cells were transferred to 12-well plates. The mStAR reporter and expression plasmids were transfected at the indicated concentration for 6 hr with Lipofectamine 2000 (Invitrogen, Urlington, ON, Canada). After a set time, cell were lysed in 100 uL reporter lysis buffer (Promega Corp, San Luis Obispo, CA, USA) and assayed for luciferase activity using a lumat LB 9507 luminometer (EG&G, Berthold, Germany).
For immunohistochemistry, 4 µm sections were cut using a Jung microtome (Leica, Heerbrugg, Switzerland). Sections were deparaffinized in xylene and hydrated in graded ethanol, followed by antigen retrieval in sodium citrate buffer. Sections were blocked in normal serum (5%) for 60 minutes and incubated with primary antibody at 4℃ overnight. After washing with Tris-buffered saline+0.05% Tween-20, Texas-red and fluorescein isothiocyanate-labeled donkey secondary antibodies (1:200, Jackson Immuno Research Lab) were added, and sections were incubated for 2 hr at room temperature. The slides were washed again and mounted with Vectashield medium (Vector Laboratories, Burlingame, CA, USA). A Zeiss Axioskop (Thornwood, NY, USA) was used to visualize the cells. Primary antibodies included rabbit polyclonal Foxo1 (1:500, SC-67140; Santa Cruz, CA, USA), Foxo3 (1:500, F2178; Sigma, MO, USA), and Foxo4 (1:200, A8975, Sigma) with goat anti-GATA-4 (1:200, Santa Cruz, CA, USA).
Cells were lysed in radioimmunoprecipitation assay buffer (20 mM Tris pH 7.5, 2 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 0.5% phosphatase inhibitor, 0.5% protease inhibitors). Fifty grams of protein was separated on 10% sodium dodecyl sulfate gels, followed by semi-dry Western blotting. Subsequently, membranes were blocked with 5% bovine serum albumin (BSA) in TBST (50 mM Tris-HCl, pH 7.5 and 150 mM NaCl containing 0.05% Tween 20).19 The blots were probed overnight with Foxo3 (1:1000, Sigma F-2178), AKT (1:1000, Cell Signaling 4691), pAKT (1:1000, Cell Signaling 4060), and phospho-Foxo3 (1:1000, Cell Signaling 2599) in 5% BSA containing TBST (10 mM Tris pH 8.0, 150 mM NaCl, 0.05% Tween-20). After incubating with an anti-rabbit secondary antibody coupled with horseradish peroxidase, the immunocomplexes were visualized with enhanced chemiluminescence.
Quantitative data are expressed as mean standard deviation. Student's t-test (two-tails) was performed using SPSS 10.0 software (SPSS Inc., Chicago, IL, USA), and all p-values<0.05 were considered statistically significant. Data are presented as the mean±standard error of the mean.
We investigated the expression of Foxo3 using double-immunostaining with GATA4, which is expressed in Sertoli cells, steroidogenic Leydig cells, and other testicular somatic cells in the testes.20 As shown in Fig. 1, Foxo3 expression was abundant in spermatogonial cytoplasm during the pre-pubertal period but was undetected after puberty. Foxo3 expression in Leydig cells, however, began to appear at 3 weeks after birth and became more widespread. Most Foxo3 protein was detected in the nucleus at 3-4 weeks after birth. At 5 and 12 weeks, however, it was localized in both the nucleus and cytoplasm, which suggests active shuttling between these compartments. Foxo3 localization in Leydig cells was regulated by age and seemed to be related to its function.
As Foxo3 showed active shuttling in the Leydig cells around puberty, we hypothesized that LH hormone could be a major regulator of Foxo3 function, since Leydig cells are the primary site of LH hormone activity. Post-translational modification is a major mechanism that regulates the localization of Foxo proteins. Among various post-translational modifications, PI3K dependent phosphorylation of Foxo3 at Thr32, Ser253, and Ser315 residues is key regulator of excluding Foxo protein from the nucleus. We looked into the phosphorylation of Thr32 of Foxo3 by hCG in the R2C cells since LH and hCG are nearly identical at the molecular level. R2C cells treated with hCG exhibited increased levels of phosphorylated AKT at 30 min, which decreased thereafter. Foxo3 phosphorylation followed a similar pattern. This indicates that hCG stimulates the PI3K pathway to regulate the phosphorylation of Foxo3. To confirm this suspicion, we investigated the phosphorylation of Foxo3 after blocking the PI3K pathway by treatment with 20 uM of LY294002. Blocking the PI3K pathway decreased hCG-mediated phosphorylation of Foxo3 and AKT. This result confirmed that hCG/LH influences the phosphorylation of Foxo3 via the PI3K pathway, mediating whether Foxo3 stays in the cytoplasm or in the nucleus.
To elucidate the role of FOXO3 in Leydig cells, FOXO3 expression was confirmed in the Leydig cell lines R2C and MA10 (Fig. 3A). Both cell lines expressed FOXO3 protein, but levels thereof were higher in R2C cells. Because the main function of Leydig cells is to produce testosterone, hormone levels were measured in R2C cells over-expressing FOXO3. Adenovirus was used to express WT- and TM-FOXO3. TM-FOXO3 has alanine residues at each of the three AKT phosphorylation sites and is constitutively active in the nucleus.17 Over-expression of WT-FOXO3 decreased testosterone levels in R2C cells, which decreased further following TM-FOXO3 over-expression (Fig. 3B). This indicates that FOXO3 negatively regulates testosterone synthesis and is more active in the nucleus.
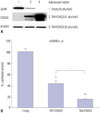
To determine how FOXO3 decreases testosterone levels in R2C cells, we investigated StAR protein levels by TM-FOXO3. In testosterone production, cholesterol transportation to the inner membrane of mitochondria is a rate-limiting step. StAR is located in mitochondria and plays a key role in steroid hormone synthesis by transporting cholesterol from the outer mitochondrial membrane to the inner membrane and converting it to pregnelone.21 TM-FOXO3 expression decreased StAR protein levels in a dose-dependent manner (Fig. 4A). To investigate whether FOXO3 directly regulates StAR gene expression, a reporter vector system was used. A mouse StAR reporter vector containing 2730 bps of the StAR promoter sequence was constructed. Cotransfecting WT FOXO3 with the reporter vector decreased mStAR promoter activity, and TM FOXO3 further decreased the reported activity (Fig. 4B). This finding corresponds with those for StAR protein levels, reflecting that FOXO3 directly regulates StAR gene expression.
While the role of Foxo3 in the female reproductive system has been widely studied and clarified, little is known about the involvement of Foxo3 in male gonad development. In this study, the presence and location of Foxo3 transcription factors in mouse Leydig cells, the regulation of its location, and its role in testosterone production were identified. We observed that Foxo3 is mainly located within the testicles from birth to PPD 5. However, after PPW 3, Foxo3 was mainly located in Leydig cells, and its expression pattern depended on age. At PPW 3 and 4, most Foxo3 was detected in the nucleus. Later, it was detected in cytoplasm and the nucleus, indicating that Foxo3 localization is regulated in Leydig cells. Thus, Foxo3 location plays an important role in the activity of Leydig cells. The importance of Foxo protein in male reproductive system is also relfected by the fact that Foxo1 and Foxo4 are also detected in the testes (data not shown). Indeed, the importance of Foxo1 on spermatogonium has been emphasized in a previous study in which germ line specific Foxo1 knock out mice showed defects in proliferative expansion, and thus, testes were small by PPD 21 due to spermatogonial arrest and failure of meiotic initiation.14
Nevertheless, Foxo3 was detected in both the cytoplasm and nucleus at 5-12 weeks after birth in Leydig cells, showing active shuttling between the nucleus and the cytoplasm. Therefore, we hypothesized that puberty hormone may be involved in regulating gonadal development. Gonadotropins FSH and LH were expected to regulate Foxo3 localization because these gonadotropins are secreted by the pituitary and act on the ovaries and testes to initiate sexual maturation and to maintain a cyclic reproductive function.2223 Among gonadotropins, LH was a candidate factor for on Foxo3 localization in Leydig cells. In females, steroidogenesis is primarily controlled by LH in Theca cells.222425 LH stimulates Theca cells to produce testosterone and maintains progesterone production by inducing the genes involved in steroidogenesis: cytochrome P450 side-chain cleavage enzyme (CYP11A1), 3β-hydroxysteroid dehydrogenase, 17α-hydroxylase/C17-20 lyase cytochrome P450 (CYP17A1), and StAR protein.2627282930 In bovine Theca cells, Akt is constitutively expressed, but it is gradually phosphorylated through LH exposure.31 AKT signaling by LH does not only influence females, but also male hormone synthesis. In primary cultures of immature rat Leydig cells, LH/chorionic gonadotropin stimulated ERK1/2 and Akt phosphorylation.253233 This result was replicated in our study (Fig. 2A). hCG treatment not only increased pAKT levels, but also pFoxo3 levels in 30 min. Activated Akt signaling by LH/hCG can increase Foxo3 phosphorylation at Thr32, Ser253, and Ser315, causing Foxo3 to be transported from the nucleus to cytoplasm. This finding indicates that LH released from the pituitary regulates Foxo3 localization. As PI3K pathway activates AKT signaling pathway, we blocked the PI3K pathway to confirm whether hCG stimulates induction of the phosphorylation of Foxo3 via the PI3K pathway. Pre-treatment of LY294002 (PI3K pathway inhibitor) reduced hCG-mediated pAKT and pFoxo3 levels, indicating the PI3K pathway regulates the localization of Foxo3 (Fig. 2B).
To determine the function of Foxo3 in Leydig cells, we measured testosterone levels in a Leydig cell line of R2C cells, as the main function of Leydig cells is testosterone synthesis. Our study revealed that FOXO3 down-regulates testosterone levels in Leydig cells (Fig. 3B). Testosterone synthesis consists of a series of steroid precursor and enzyme syntheses. The ratelimiting step in steroid synthesis is catalyzed by StAR protein, which transports cholesterol from the outer mitochondrial membrane to the inner membrane.3435 StAR protein levels decreased dramatically in a dose-dependent manner in R2C cells infected with TM-FOXO3 (Fig. 4A). Next, we observed whether mStAR promoter activity is directly down-regulated by FOXO3. FOXO3 down-regulated mStAR promoter activity, which was more significant with TM-FOXO3 expression (Fig. 4B). This result correlates with the reduced testosterone levels caused by over-expression of WT and TM-FOXO3 in R2C cells (Fig. 3B). From those results, we concluded that Foxo3 plays an important role in testosterone hormone synthesis. However, there is no reported phenotype in Foxo3 KO male mice. It seems to be that Leydig cells not only express Foxo3 but also Foxo4 (data not shown). Foxo4 seems to have similar function in Leydig cells.
Foxo3 may be regulated by other signaling pathways, such as insulin like growth factor (IGF)-1 signaling. In the testes, IGF-1 reaches its maximum expression at PPW 4 when pubertal testosterone rises.36 Growth factor have been shown to activiate AKT, which leads to the phosphorylation of Foxo3 at its 14-3-3 binding site and determines Foxo3 relocalization to the cytoplasm.9 This result with our observations led us to hypothesize that during the first four weeks, IGF-1 phosphorylates Foxo3, trapping it in the cytoplasm through interactions with 14-3-3. Once IGF-1 has reached its peak expression and decreases, LH signaling-mediating Foxo3 may become involved in the regulation of gonadal development by regulating testosterone hormone.
In summary, this study investigated Foxo3 localization and its role in the testes. Foxo3 is expressed in the testes, but its expression sites differ according to age. Foxo3 expression in Leydig cells appears in the nucleus before puberty, and it shows active shuttling in between the nucleus and the cytoplasm after puberty. We confirmed the role of Foxo3 in Leydig cells as a negative regulator for testosterone hormone by reducing StAR gene expression. The fact that phosphorylated Foxo3 by hCG/LH suggests a possible mechanism that LH stimulates testosterone levels via PI3K-dependent inhibition of Foxo3 action in Leydig cells.
Figures and Tables
Fig. 1
Foxo3 is detected in Leydig cells. In C57/BL6 male mice, testes were isolated at postpartum days (PPD) 1 and 5 and postpartum weeks (PPW) 3, 4, 5, and 12. Double-immunostaining was performed using each rabbit polyclonal Foxo3 with goat anti-GATA4 and observed under a laser scanning microscope, ×400 magnification. Scale bar indicates 200 um. FITC, fluorescein isothiocyanate; DAPI, 4',6-diamidino-2-phenylindole.
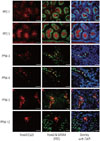
Fig. 2
LH/hCG regulates Foxo3 localization. (A) R2C cells were cultured in the serum-starved condition for 24 hr and stimulated with 10 IU of hCG for 10, 30, and 60 minutes. (B) To block the PI3K pathway, 20 uM of LY294002 was pretreated for 2 hr and then 10 IU of hCG was added for 30 min. Cell lysates were extracted and western blots were performed. The grape data was presented as the mean (±SEM) of triplicated experiments from three independent experiments and are presented as fold changes related to 0 min treatment in (A) and no-treatment in (B). Significant differences between control are denoted as *p<0.05, **p<0.01, †p<0.05, and ††p<0.01 compared btween 30 min and 60 min. Similar results were obtained in three independent experiments. LH, luteinizing hormone; hCG, human chorionic gonadotropin; PI3K, phosphoinositide 3-kinase; SEM, standard error of the mean; NS, not significant.

Fig. 3
FOXO3 over-expression decreases testosterone levels in Leydig cell lines. (A) We confirmed the Foxo3 levels in MA10 and R2C cells. To elucidate the role of Foxo3 in the nucleus, we constructed a constitutively active form of human FOXO3 by replacing the three AKT phosphorylation sites (T32, S253, and S315) with alanine. (B) Testosterone levels were measured in R2C cells infected with Ad-LacZ (empty), WT-FOXO3, or TM-FOXO3 adenovirus (5 pfu/cell for 48 hr). Testosterone levels decreased by WT-FOXO3 infection and more significantly by TM-FOXO3. *p<0.05, **p<0.01 vs. empty or WT-FOXO3 infection sample. Similar results were obtained in three independent experiments. WT, wild type; TM, triple mutant.
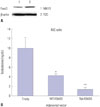
Fig. 4
Constitutively active FOXO3 downregulates StAR protein levels by directly inhibiting StAR gene expression. (A) StAR protein levels were measured in R2C cells infected with Ad-LacZ (empty) or TM-FOXO3 (1 or 5 pfu/cell) for 48 hr. Constitutively active FOXO3 decreases StAR protein level in a dose-dependent manner in R2C cells. Similar results were obtained from three independent experiments. (B) FOXO3 down-regulates mStAR promoter activity. mStAR promoter activity was measured by co-transfecting mStAR-Luc plasmid with empty, WT-FOXO3, or TM-FOXO3 vectors in HEK293 cells (*p<0.05 and **p<0.01 vs. empty, †p<0.05 compared between 30 min and 60 min). Similar results were obtained in three independent experiments. StAR, steroidogenic acute regulatory; WT, wild type; TM, triple mutant; mStAR, mouse steroidogenic acute regulatory protein.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean government (MEST) (No. 2011-0016060).
References
2. Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003; 125:769–784.
3. Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, Le-Meur M, et al. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998; 95:13612–13617.

4. Purvis K, Hansson V. Hormonal regulation of Leydig cell function. Mol Cell Endocrinol. 1978; 12:123–138.

5. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci U S A. 2004; 101:17294–17299.

6. Jasti P, Dunaif A. Reproduction and metabolism: insights from polycystic ovary syndrome. Endocrinol Metab. 2012; 27:180. 190.

7. Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007; 120(Pt 15):2479–2487.
8. Lee EJ, Anderson LM, Thimmapaya B, Jameson JL. Targeted expression of toxic genes directed by pituitary hormone promoters: a potential strategy for adenovirus-mediated gene therapy of pituitary tumors. J Clin Endocrinol Metab. 1999; 84:786–794.
9. Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta. 2011; 1813:1938–1945.

10. Richards JS, Sharma SC, Falender AE, Lo YH. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol. 2002; 16:580–599.
11. Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, et al. Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J Biol Chem. 2005; 280:9135–9148.
12. Hosaka T, Biggs WH 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004; 101:2975–2980.

13. Matsuda F, Inoue N, Maeda A, Cheng Y, Sai T, Gonda H, et al. Expression and function of apoptosis initiator FOXO3 in granulosa cells during follicular atresia in pig ovaries. J Reprod Dev. 2011; 57:151–158.

14. Goertz MJ, Wu Z, Gallardo TD, Hamra FK, Castrillon DH. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J Clin Invest. 2011; 121:3456–3466.

15. Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003; 301:215–218.

16. Lee EJ, Kim JM, Lee MK, Jameson JL. Splice variants of the forkhead box protein AFX exhibit dominant negative activity and inhibit AFXalpha-mediated tumor cell apoptosis. PLoS One. 2008; 3:e2743.
17. Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003; 278:29619–29625.

18. Hong ZY, Lee HJ, Shin DY, Kim SK, Seo M, Lee EJ. Inhibition of Akt/FOXO3a signaling by constitutively active FOXO3a suppresses growth of follicular thyroid cancer cell lines. Cancer Lett. 2012; 314:34–40.

19. Kim JW, Kim HS, Kim SD, Park JY. Insulin Phosphorylates Tyrosine Residue 464 of Tub and Translocates Tubby into the Nucleus in HIRcB Cells. Endocrinol Metab (Seoul). 2014; 29:163–168.
20. Ketola I, Pentikäinen V, Vaskivuo T, Ilvesmäki V, Herva R, Dunkel L, et al. Expression of transcription factor GATA-4 during human testicular development and disease. J Clin Endocrinol Metab. 2000; 85:3925–3931.

21. Aspden WJ, Rodgers RJ, Stocco DM, Scott PT, Wreford NG, Trigg TE, et al. Changes in testicular steroidogenic acute regulatory (STAR) protein, steroidogenic enzymes and testicular morphology associated with increased testosterone secretion in bulls receiving the luteinizing hormone releasing hormone agonist deslorelin. Domest Anim Endocrinol. 1998; 15:227–238.

22. Wang XX, Wu ZY. [Prediction of ovulation]. Zhonghua Fu Chan Ke Za Zhi. 1990; 25:86–88. 124
23. Bäckström CT, McNeilly AS, Leask RM, Baird DT. Pulsatile secretion of LH, FSH, prolactin, oestradiol and progesterone during the human menstrual cycle. Clin Endocrinol (Oxf). 1982; 17:29–42.

24. Erickson GF, Ryan KJ. Stimulation of testosterone production in isolated rabbit thecal tissue by LH/FSH, dibutyryl cyclic AMP, PGE2alpha, and PGE2. Endocrinology. 1976; 99:452–458.

25. Palaniappan M, Menon KM. Human chorionic gonadotropin stimulates theca-interstitial cell proliferation and cell cycle regulatory proteins by a cAMP-dependent activation of AKT/mTORC1 signaling pathway. Mol Endocrinol. 2010; 24:1782–1793.

26. Bogovich K, Richards JS. Androgen biosynthesis in developing ovarian follicles: evidence that luteinizing hormone regulates thecal 17 alpha-hydroxylase and C17-20-lyase activities. Endocrinology. 1982; 111:1201–1208.

27. Magoffin DA, Kurtz KM, Erickson GF. Insulin-like growth factor-I selectively stimulates cholesterol side-chain cleavage expression in ovarian theca-interstitial cells. Mol Endocrinol. 1990; 4:489–496.

28. Magoffin DA, Weitsman SR. Differentiation of ovarian theca-interstitial cells in vitro: regulation of 17 alpha-hydroxylase messenger ribonucleic acid expression by luteinizing hormone and insulin-like growth factor-I. Endocrinology. 1993; 132:1945–1951.

29. Magoffin DA, Weitsman SR. Insulin-like growth factor-I stimulates the expression of 3 beta-hydroxysteroid dehydrogenase messenger ribonucleic acid in ovarian theca-interstitial cells. Biol Reprod. 1993; 48:1166–1173.

30. Mizutani T, Yazawa T, Ju Y, Imamichi Y, Uesaka M, Inaoka Y, et al. Identification of a novel distal control region upstream of the human steroidogenic acute regulatory protein (StAR) gene that participates in SF-1-dependent chromatin architecture. J Biol Chem. 2010; 285:28240–28251.

31. Fukuda S, Orisaka M, Tajima K, Hattori K, Kotsuji F. Luteinizing hormone-induced Akt phosphorylation and androgen production are modulated by MAP Kinase in bovine theca cells. J Ovarian Res. 2009; 2:17.

32. Shiraishi K, Ascoli M. Lutropin/choriogonadotropin stimulate the proliferation of primary cultures of rat Leydig cells through a pathway that involves activation of the extracellularly regulated kinase 1/2 cascade. Endocrinology. 2007; 148:3214–3225.

33. Martinelle N, Holst M, Söder O, Svechnikov K. Extracellular signal-regulated kinases are involved in the acute activation of steroidogenesis in immature rat Leydig cells by human chorionic gonadotropin. Endocrinology. 2004; 145:4629–4634.

34. Stocco DM, Clark BJ. Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem Pharmacol. 1996; 51:197–205.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download