Abstract
Purpose
During sedated esophagogastroduodenoscopy (EGD), patients may not be able to perform inspiration, which is necessary to examine the esophagogastric junction. Therefore sedation may affect diagnosis of gastroesophageal reflux-related findings. The aim of our study was to investigate the effect of sedation on diagnosis of gastroesophageal reflux-related findings during EGD.
Materials and Methods
This retrospective study evaluated 28914 patients older than 20 years who underwent EGD at our institution between January 2011 and December 2011. Ultimately, 1546 patients indicated for EGD for health check-up and symptom evaluation were included.
Results
There were 18546 patients who had diagnostic EGD: 10471 patients (56%) by non-sedated EGD and 8075 patients (43%) by sedated EGD. After statistical adjustment for age, sex, and body mass index, minimal change esophagitis, and hiatal hernia were significantly less frequently observed in the sedated EGD group [odds ratio (OR), 0.651; 95% confidence interval (CI), 0.586 to 0.722 and OR, 0.699; 95% CI, 0.564 to 0.866]. Nevertheless, there was no significant difference in other findings at the gastroesophageal junction, such as reflux esophagitis with Los Angeles classification A, B, C, and D or Barrett's esophagus, between the two groups. Similarly, there were no differences in early gastric cancer, advanced gastric cancer, and gastric ulcer occurrence.
In South Korea, health check-ups for screening purposes have recently become quite popular. Due to a high incidence of gastric cancer, esophagogastroduodenoscopy (EGD) is offered biannually in individuals older than 40 years via Korea's National Health Insurance check-up program.
The prevalence of gastroesophageal reflux disease in Asia has increased. Prior to 2000, the prevalence of reflux esophagitis diagnosed on EGD was less than 10%,1 and since then, has gradually increased up to 13.8%.23 In a national study in Korea from 2005 to 2008, the prevalence of reflux esophagitis was reported as 7.91% based on endoscopic findings at health check-ups (n=25536).4
Use of sedated EGD has increased to control patient anxiety and pain during EGD. Commonly, patients are allowed to opt for sedation if it is not contraindicated, since sedation improves patient tolerance during the procedure. Many studies have been published about sedation regimens during EGD, as well as associated risks and benefits, with appropriate safety measures and monitoring practices.56 While EGD can be performed correctly under sedation, patients may not be able to perform deep inspiration when examining the esophagogastric junction (EGJ). Thus, sedation likely affects the diagnosis of gastroesophageal reflux-related findings. The EGJ is functionally and anatomically complex and is not easily evaluated. Although the methods, degrees, and duration of sedated EGD have been studied extensively, there have been no reports on the influence of sedation on diagnosis of disease during EGD. Therefore, we elected to study the implications of sedation on the diagnosis of upper gastrointestinal disease, particularly gastroesophageal reflux-related findings, during EGD.
We performed a retrospective review of data from 28914 patients older than 20 years who underwent EGD at the Severance Hospital, Yonsei University College of Medicine, in Seoul, Korea from January 2011 to December 2011. A total of 28914 patients received an EGD during the study period. We excluded therapeutic EGD, such as endoscopic mucosal resection, endoscopic submucosal dissection, percutaneous endoscopic gastrostomy, and hemostasis for gastrointestinal bleeding; emergency EGD; and scheduled EGD after upper gastrointestinal malignancy treatment. Consequently, the study population comprised 18546 patients who received a diagnostic EGD and fulfilled the inclusion criteria. Of these, 10471 patients (56.4%) underwent non-sedated EGD and 8075 patients (43.5%) underwent sedated EGD (Fig. 1). Data related to the patient's characteristics and endoscopic findings were extracted.
EGD was performed by experienced endoscopists who had each performed more than 3000 upper endoscopies and well-trained endoscopists who performed at least more than 130 cases, which are minimum quality requirements for competence according to a specialist medical society.7
EGD was accomplished in the left lateral decubitus position, and patients received topical pharyngeal anesthesia with lidocaine spray. All patients were assessed with a history and physical examination prior to administering sedation to identify factors that may increase the risk of an adverse outcome. The sedated EGD was performed by low-dose propofol sedation. Before initiation of EGD, a 20 gauge (1.0 mm) i.v. cannula was placed in the patient's forearm for propofol injection. An additional 20 mg of propofol was injected if the target level was not obtained.6 Low-dose propofol was administered for endoscopic sedation by nurses supervised by the endoscopists. Both the endoscopists and nurses had basic cardiac life support certification.8 The target level of sedation was conscious sedation that maintained both ventilator and cardiovascular function, and allowed patients to make a purposeful response to verbal or tactile stimulation. Patient monitoring was performed in accordance with the recommendations of the American Society for Gastrointestinal Endoscopy.6
We used a gastrointestinal videoscope (GIF-Q260, GIF-H260; Olympus Optical Co. Ltd., Tokyo, Japan) with an outer diameter of 9.2, 9.8 mm by an oral approach. Endoscopic examination of the EGJ was performed using a high-definition white-light endoscope without specialized equipment, such as chromoendoscopy or narrow band imaging, and was inspected as described below. We asked the patients to hold their breathing after deep inspiration. Then, we observed the EGJ when it was most widely opened during peristalsis (Fig. 2). The remainder of the endoscopic examination was performed according to the department's standard operating procedure. The EGJ was defined to include the squamocolumnar junction, the proximal margin of gastric folds, the distal end of the palisade zone, and the location of pinchcock. Endoscopic findings of reflux esophagitis in the lower esophagus were classified according to the Los Angeles (LA) classification as grades A to D, and were based on the longest length of a mucosal break and the confluence of erosion. Six endoscopic criteria were used to define minimal change esophagitis: erythema, blurring of the Z-line, white turbid discoloration, decreased vascularity, friability, and edema or accentuation of the mucosal fold.91011
This study protocol was approved by the Institutional Review Board of Yonsei University Hospital (4-2012-0124) and the study was conducted in accordance with the Helsinki Declaration.
Continuous variables are expressed as the mean±standard deviation. The chi-square test was used to compare categorical variables, and a t-test was used to compare continuous variables. Logistic regression adjusted for age, sex, and body mass index (BMI) was used to assess the effect of sedation on the diagnosis of gastroesophageal reflux-related findings during EGD. A p-value of less than 0.05 was considered statistically significant. Statistical analysis was conducted using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Among the 18546 patients, 9369 (50.5%) were men and 9177 (49.4%) were women. The mean age was 56 years (range: 20-96 years, SD: 13.9) and the mean BMI was 23.1 (SD: 3.4). Table 1 shows the characteristics of the study population. The data revealed significant differences in clinical characteristics, such as sex, age, BMI, hypertension, and diabetes mellitus. However, as data on smoking, alcohol, hypertension, and diabetes mellitus were missing in about half of the patients, we do not include these variables when comparing and analyzing sedation effects during EGD. We adjusted for age, sex, and BMI when evaluating the collected data.
Table 2 demonstrates the reasons for diagnostic EGD, which we classified into evaluation for symptoms and previous findings and a regular health check-up. The common purpose of diagnostic EGD was to evaluate gastrointestinal symptoms and previous EGD findings. There were no significant differences between the sedated and non-sedated groups regarding the purposes of diagnostic EGD.
Table 3 shows differences in the endoscopic findings of the EGJ observed between the sedated EGD group and non-sedated EGD group. Sedated EGD was significantly associated with less frequent detection of minimal change esophagitis [odds ratio (OR), 0.651; 95% confidence interval (CI), 0.586 to 0.722] and hiatal hernia (OR, 0.699; 95% CI, 0.564 to 0.866) at the EGJ. Likewise, other findings at the EGJ, such as reflux esophagitis of LA classification A (OR, 0.949; 95% CI, 0.807 to 1.116), B (OR, 0.782; 95% CI, 0.589 to 1.038), C (OR, 0.872; 95% CI, 0.447 to 1.700), and D (OR, 0.639; 95% CI, 0.226 to 1.805), and Barrett's esophagus (OR, 0.794; 95% CI, 0.586 to 1.076), were also less frequently detected, although the differences therein between the two groups was not significant.
Findings in the stomach, such as early gastric cancer (OR, 1.145; 95% CI, 0.995 to 1.317), advanced gastric cancer (OR, 0.896; 95% CI, 0.768 to 1.044), and gastric ulcer (OR, 0.963; 95% CI, 0.857 to 1.083), did not differ between the two groups (Table 4).
Conscious sedation is often performed in diagnostic and uncomplicated therapeutic EGD. The purpose of this study was to investigate the influence of sedation on the diagnosis of gastroesophageal reflux-related findings during EGD. After adjusting for sex, age, and BMI, diagnoses of minimal change esophagitis and hiatal hernia were more frequent for non-sedated EGD than sedated EGD (12.7% vs. 10.0% and 3.3% vs. 1.9%, respectively). Reflux esophagitis of LA classification A to D was not significantly different between the sedated and non-sedated EGD groups in this study.
There are two possible explanations for the greater detection of a minimal change esophagitis in non-sedated EGD. One reflects the sedation itself during EGD, while the other involves the effect of propofol on esophageal motility. Sedation impacts EGJ observation in the following two ways: first, sedation can provide endoscopists enough time to observe the EGJ as long as they want, compared to non-sedated EGD. Second, sedation hampers performing the procedure at the EGJ adequately due to a lack of cooperation between the endoscopist and the patient with regard to breathing, which was observed in this study.
Deep inspiration generates negative pressure in the thoracic cavity. In this interim, intraabdominal pressure increases and the diaphragm flattens.12 Thus, deep inspiration facilitates the extension of the esophagus so that the EGJ can be observed easily. This may have caused the difference in the diagnosis of the gastroesophageal reflux-related finding between the two groups in this study.
When propofol is used at a lower dose (0.3 mg/kg), there is no alteration in pressure of the lower esophageal sphincter. However, there is an increase in pressure in young patients (less than 30) after a high dose (0.9 mg/kg) of propofol. In our study, almost all patients were older than forty; therefore, propofol itself would not be expected to affect the motility of the esophagus.13
Minimal change esophagitis was defined as a whitish or reddish, edematous change and erosion that was not a mucosal break. The interobserver agreement for this lesion is low in comparison to that of reflux esophagitis LA classification A to D,14 so the diagnosis of this lesion must made more precisely and carefully. Although minimal changes are one of the endoscopic findings of non-erosive reflux disease, the clinical significance of these minimal changes is controversial. In our previous report, most endoscopic findings indicating minimal changes were not related with symptoms of gastroesophageal reflux disease. Together with previous studies about minimal change esophagitis, diagnosis of minimal change esophagitis should be reconsidered because of low interobserver agreement, as well as a lack of clinical meaning and variability in the methods of examination.
In Asia, several studies have been performed on the prevalence of endoscopy-based reflux esophagitis. The prevalence of endoscopic esophagitis is generally less than 10%, although it has been increasing in recent years.15161718 In our study, the prevalence of endoscopic reflux esophagitis was found to be 5.7%, which is similar to the 7.9% prevalence rate reported in 200517 and the 3.4% prevalence rate in 2001 for Koreans,19 although lower than the prevalence observed in the West.20 Barrett's esophagus has a very low prevalence in Asia of less than 0.03% of the general population2122 and 2% of upper gastrointestinal symptoms.3 The prevalence of Barrett's esophagus was 1.4% in this study. The reason for this relatively high prevalence was that only 21.8% of EGD was conducted as a routine health check-up, while the majority was conducted for the evaluation of upper gastrointestinal symptoms, diagnosis of disease, and after receiving a referral to our hospital. Although there was a difference (OR, 0.794; 95% CI, 0.586 to 1.076) between the sedated and non-sedated EGD groups, it was not statistically significant (p=0.136).
One of the risk factors of Barrett's esophagus is hiatal hernia. Hiatal hernia is defined as the persistent or recurrent herniation of parts of the stomach through the esophageal hiatus into the chest cavity.23 For accurate observation of a hiatal hernia, the endoscopist requires proper air inflation in the stomach and the patient's cooperation in breath control. Therefore, sedation affects the diagnosis of gastroesophageal reflux-related findings, such as minimal change esophagitis and hiatal hernia, as we found in this study.
Finally, there are several limitations in our study. Firstly, this study was performed retrospectively. Although all endoscopic data were recorded prospectively and properly selected, the chance of bias cannot be negated. Secondly, confounding factors to have a potential effect on examination of EGJ including proton pump inhibitor (PPI) medication, obesity, smoking status, and chronic airway diseases, such as chronic obstructive pulmonary disease and asthma, to cause airflow limitation were not thoroughly evaluated. This problem can be solved by future prospective studies. Thirdly, this study was performed at a tertiary referral hospital with a high prevalence of gastric cancer compared to the general population. Therefore, it is difficult to generalize our findings regarding the potential effect of sedation on diagnoses.
In this study, we have shown that sedation during the EGD can affect the sensitivity of diagnosis of minimal change esophagitis and hiatal hernia. Sedation might interfere with detailed examination of the EGJ, compared to non-sedative EGD. However, clinically significant findings such as reflux esophagitis of LA classification A to D and Barrett's esophagus were not influenced by sedation. Therefore, we suggest that patient's sedation status should be considered while evaluating minimal change esophagitis or hiatal hernia.
Figures and Tables
Fig. 2
Typical findings at the esophagogastric junction in the non-sedated EGD group (A) and the sedated EGD group (B). EGD, esophagogastroduodenoscopy.
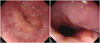
Table 1
Clinical Characteristics of the 18546 Patients

Table 2
Purposes of EGD among the Study Cohort

| Evaluation for symptoms and previous findings | Regular health check-up | Total EGD | p value | |
|---|---|---|---|---|
| Non-sedated EGD | 8134 (77.7) | 2337 (22.3) | 10471 (100) | 0.051 |
| Sedated EGD | 6369 (78.9) | 1706 (21.1) | 8075 (100) |
Table 3
Endoscopic Findings at the Esophagogastric Junction during Sedated and Non-Sedated EGD

Table 4
Endoscopic Findings at the Stomach during Sedated and Non-Sedated EGD

ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2011 (6-2011-0103).
References
1. Goh KL, Chang CS, Fock KM, Ke M, Park HJ, Lam SK. Gastro-oesophageal reflux disease in Asia. J Gastroenterol Hepatol. 2000; 15:230–238.

2. Inamori M, Togawa J, Nagase H, Abe Y, Umezawa T, Nakajima A, et al. Clinical characteristics of Japanese reflux esophagitis patients as determined by Los Angeles classification. J Gastroenterol Hepatol. 2003; 18:172–176.

3. Rosaida MS, Goh KL. Gastro-oesophageal reflux disease, reflux oesophagitis and non-erosive reflux disease in a multiracial Asian population: a prospective, endoscopy based study. Eur J Gastroenterol Hepatol. 2004; 16:495–501.

4. Shim KN, Hong SJ, Sung JK, Park KS, Kim SE, Park HS, et al. Clinical spectrum of reflux esophagitis among 25,536 Koreans who underwent a health check-up: a nationwide multicenter prospective, endoscopy-based study. J Clin Gastroenterol. 2009; 43:632–638.

5. Thomson A, Andrew G, Jones DB. Optimal sedation for gastrointestinal endoscopy: review and recommendations. J Gastroenterol Hepatol. 2010; 25:469–478.

6. Standards of Practice Committee. Lichtenstein DR, Jagannath S, Baron TH, Anderson MA, Banerjee S, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008; 68:205–216.

7. Eisen GM, Baron TH, Dominitz JA, Faigel DO, Goldstein JL, Johanson JF, et al. Methods of granting hospital privileges to perform gastrointestinal endoscopy. Gastrointest Endosc. 2002; 55:780–783.

8. Horiuchi A, Nakayama Y, Hidaka N, Ichise Y, Kajiyama M, Tanaka N. Low-dose propofol sedation for diagnostic esophagogastroduodenoscopy: results in 10,662 adults. Am J Gastroenterol. 2009; 104:1650–1655.

9. Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996; 111:85–92.

10. Hongo M. Minimal changes in reflux esophagitis: red ones and white ones. J Gastroenterol. 2006; 41:95–99.

11. Kim JH, Park H, Lee YC. MIGHT study group. Is minimal change esophagitis really part of the spectrum of endoscopic findings of gastroesophageal reflux disease? A prospective, multicenter study. Endoscopy. 2011; 43:190–195.

12. Block B. Endoscopy of the upper GI tract. New York, NY: Thieme Medical Publishers, Incorporated.;2004. p. 32–33.
13. de Leon A, Ahlstrand R, Thörn SE, Wattwil M. Effects of propofol on oesophageal sphincters: a study on young and elderly volunteers using high-resolution solid-state manometry. Eur J Anaesthesiol. 2011; 28:273–278.

14. Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999; 45:172–180.

15. Chang CS, Poon SK, Lien HC, Chen GH. The incidence of reflux esophagitis among the Chinese. Am J Gastroenterol. 1997; 92:668–671.
16. Watanabe T, Urita Y, Sugimoto M, Miki K. Gastroesophageal reflux disease symptoms are more common in general practice in Japan. World J Gastroenterol. 2007; 13:4219–4223.

17. Kim N, Lee SW, Cho SI, Park CG, Yang CH, Kim HS, et al. The prevalence of and risk factors for erosive oesophagitis and non-erosive reflux disease: a nationwide multicentre prospective study in Korea. Aliment Pharmacol Ther. 2008; 27:173–185.

18. Kim KM, Cho YK, Bae SJ, Kim DS, Shim KN, Kim JH, et al. Prevalence of gastroesophageal reflux disease in Korea and associated health-care utilization: a national population-based study. J Gastroenterol Hepatol. 2012; 27:741–745.

19. Lee SJ, Song CW, Jeen YT, Chun HJ, Lee HS, Um SH, et al. Prevalence of endoscopic reflux esophagitis among Koreans. J Gastroenterol Hepatol. 2001; 16:373–376.

20. Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005; 54:710–717.

21. Kim JH, Rhee PL, Lee JH, Lee H, Choi YS, Son HJ, et al. Prevalence and risk factors of Barrett's esophagus in Korea. J Gastroenterol Hepatol. 2007; 22:908–912.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download