Abstract
Purpose
To investigate the effects of anthocyanins extracted from black soybean, which have antioxidant activity, on apoptosis in vitro (in hormone refractory prostate cancer cells) and on tumor growth in vivo (in athymic nude mouse xenograft model).
Materials and Methods
The growth and viability of DU-145 cells treated with anthocyanins were assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and apoptosis was assessed by DNA laddering. Immunoblotting was conducted to evaluate differences in the expressions of p53, Bax, Bcl, androgen receptor (AR), and prostate specific antigen (PSA). To study the inhibitory effects of anthocyanins on tumor growth in vivo, DU-145 tumor xenografts were established in athymic nude mice. The anthocyanin group was treated with daily oral anthocyanin (8 mg/kg) for 14 weeks. After 2 weeks of treatment, DU-145 cells (2×106) were inoculated subcutaneously into the right flank to establish tumor xenografts. Tumor dimensions were measured twice a week using calipers and volumes were calculated.
Results
Anthocyanin treatment of DU-145 cells resulted in 1) significant increase in apoptosis in a dose-dependent manner, 2) significant decrease in p53 and Bcl-2 expressions (with increased Bax expression), and 3) significant decrease in PSA and AR expressions. In the xenograft model, anthocyanin treatment significantly inhibit tumor growth.
Prostate cancer (PCa) is the most frequently diagnosed male malignancy in most industrialized countries.1 Recently, Asian countries, including Korea, have reported high PCa incidences and consequent mortality rates.2 Epidemiological studies suggest that diets rich in natural ingredients (e.g., fruits and vegetables), such as those common in Asian countries, have the potential to protect against prostate cancer.3 Western dietary habits, on the other hand, may contribute to increased prostate cancer morbidity and mortality.4 Dietary factors have been associated with increased prostate cancer incidence, thereby leading to studies of dietary modification and supplementation to prevent and treat prostate cancer.5,6
Several reports indicate that the cumulative production of reactive oxygen species through endogenous or exogenous insults (i.e., oxidative stress) may play an important role in carcinogenesis.7,8 Oxidative stress induces a cellular redox imbalance, which has been found in various cancer cells compared with normal cells, and may be related to oncogenic stimulation. DNA mutation is a critical step in carcinogenesis and elevated levels of oxidative DNA lesions have been noted in various tumors, strongly implicating such damage in the initiation of cancer.7 Furthermore, aging results from a cellular imbalance in pro-oxidants and antioxidants.9,10 Antioxidant defense mechanisms, including reactive oxygen species (ROS) detoxification enzyme activity, decline with age,11,12 shifting redox balance towards an oxidative state.
Bostwick, et al.8 evaluated the expression of antioxidant enzymes in prostate cancer tissue and found decreased expression and down-regulation of antioxidants in prostatic intraepithelial neoplasia (PIN) and prostate cancer compared with benign epithelium. There was a significant amount of cumulative oxidative DNA damage limited to the epithelium in PIN lesions and prostate cancer. Associations of aging with prostate cancer and oxidative stress show that aging-related radical-induced DNA damage is linked to prostate cancer.13,14
These findings suggest that oxidative stress is an early event in carcinogenesis and a contributory risk factor for prostate cancer. Therefore, minimizing DNA damage by decreasing oxidative stress may prevent and treat prostate cancer.
The aforementioned epidemiologic results can be attributed, in part, to diets rich in natural ingredients, which have antioxidative potencies. Therefore recent approaches to control disease have used naturally occurring dietary substances. Previously, we identified the antioxidant and apoptosis-inducing effects of anthocyanins extracted from black soybean in prostate disease associated with oxidative stress.15 We further showed that anthocyanin was a novel and potent antioxidant with diverse physiological activities. In this study, we found that anthocyanins extracted from black soybean induce apoptosis in a hormone refractory prostate cancer cell line and inhibit tumor growth in an athymic nude mouse xenograft model.
The anthocyanin used in our experiments was extracted from the seed coat of black soybean and was supplied by the Rural Development Administration, Suwon, Republic of Korea. The anthocyanin was extracted and analyzed as previous described.16 A normal prostate cell line, RWPE-1 (ATCC, Manassas, VA, USA) and an androgen-independent prostate cancer cell line, DU-145 (Korean Cell Line Bank, Seoul, Korea) were cultured in RPMI 1640 supplemented with 10% FBS, L-glutamine (300 mg/L), 25 mM HEPES, and 25 mM NaHCO3.
Cell growth and viability were evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO, USA) assay. RWPE-1 and DU-145 cells were seeded in 96-well plates (5×103 cells per well in 100 mL). After growing for 24 h in RPMI-1640 medium, the cells were treated with various concentrations (0, 30, 60, or 120 µM) of anthocyanin for 24, 48, or 72 hours to evaluate the effect of the anthocyanin on cell viability. At the end of the treatment, the medium was removed and 0.5 mg·mL-1 of MTT was added to the medium. After 4 h, DMSO (200 mL) was added to each well and the optical density was read at 570 nm. Cell sensitivity to drug treatment was expressed as the drug concentration that yielded 50% cell inhibition (IC50). All experiments were performed in triplicate.
DU-145 cells (5×105 cells mL-1) treated with vehicle only or anthocyanin (60 or 120 µM for 24 or 48 hours) and were washed with PBS. Two hundred µL of binding/lysis buffer was added, the total volume was adjusted to 400 µL, and mixed. After incubating at 15-25℃ for 10 minutes, 100 µL of isopropanol was added, and shaken. The samples were added to a filter tube attached to a collection tube and centrifuged at 800 rpm for 1 minute. Washing buffer (500 µL) was added and centrifuged at 800 rpm for 1 minute twice, and a third time at 13000 rpm for 10 seconds. Elution buffer (100 µL) was added, and centrifuged at 800 rpm for 1 deminute. Finally, electrophoresis was performed on a 1% agarose gel at 75 V for 1.5 hours, and viewed under ultraviolet light. Apoptosis of transfected and control cells was confirmed by DNA laddering assay as described.17
DU-145 cells (5×105 cells mL-1) were treated with with vehicle only or anthocyanin (60 or 120 µM for 24 or 48 hours) were extracted by centrifuging at 2000 rpm, for 5 minutes at 4℃, and lysed with 100 µL of lysis buffer for 30 minutes at room temperature (RT). The supernatant was extracted, quantified using a bicinchonic acid assay (Pierce, Rockford, IL, USA), and electrophoresed on an SDS-PAGE gel. After transfering to a nitrocellulose membrane, the membrane was blocked with 5% skim milk and then incubated overnight at 4℃ with an anti-p53 antibody (1:1000; Abcam, Cambridge, UK), an anti-Bcl-2 antibody (1:1000; Abcam, Cambridge, UK), an anti-Bax antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) an anti-PSA antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), or an anti-AR antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Membranes were washed with TBS-T (TBS, 0.1% Tween 20) and secondary anti-mouse or anti-rabbit antibodies (Invitrogen Corporation, Paisley, UK) conjugated to horseradish peroxidase were added at RT for 1 h. To adjust for loading differences, membranes were reprobed with a β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The blots were analyzed using a chemiluminescence detection system (GE Healthcare, Pittsburgh, PA, USA). Densitometric analyses were performed using Image J software (NIH, Bethesda, MD, USA).
Nicotinamide adenine dinucleotide (NAD+)/NADH ratio was quantitatively assessed by enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (BioVision, Milpitas, CA, USA). Cells were pelleted in a micro-centrifuge tube and extracted with 400 µL of NADH/NAD+ extraction buffer by freeze/thaw two cycles or by homogenization. After centrifugation, the extracted NADH/NAD+ supernatant was transferred into a labeled tube. To detect total NADH (NADH and NAD+), 50 µL of extracted samples were transferred into labeled 96-well plate. To detect NADH, NAD+ needs to be decomposed before the reaction. Thus, to decompose NAD+, 200 µL aliquot of extracted samples were transferred to eppendorf tubes and heated to 60℃ for 30 min, and then samples were cooled on ice. The samples were spinned to remove precipitates if they occurred. Fifty µL of NAD+ decomposed samples were transferred to labeled 96-well plate. After the final color was developed with the addition of 10 µL NADH developer into each well, absorbance was measured at 450 nm.
The experimental animals were 6-week-old male BALB/c nude mice, weighing 18-22 g, provided by Samtaco Bio Co. (Osan, Korea). After a 1-week adjustment period, the animals were maintained in plastic cages, with 5 animals per cage. This study was approved by the Institutional Animal Care and Use Committee of the Catholic University of Korea, Seoul St. Mary's Hospital (IRB approval no. CUMC-2011-0026-02). Experimental animals were randomly allocated into 2 groups (control and anthocyanin), each consisting of 10 animals. Animal in the anthocyanin group were treated with daily oral anthocyanin (8 mg/kg), dissolved in 1 mL of distilled water and administered through an 8 F red Rob-Nel catheter once daily for 14 weeks. Animal in the control group received vehicle in the same manner and period as the experimental group. After 2 weeks of treatment, to establish tumor xenografts, DU-145 cells (2×106) were suspended in 0.1 mL of medium and inoculated subcutaneously into the right flank of the mice in both groups. Body weights and tumor volumes were monitored twice weekly using calipers and calculated according to the formula (L×W2)/2, where L and W are the length and width, respectively.18
All experiments were performed on 3 separate cultures. All data are presented as mean±standard deviation, where p<0.05 is considered to be statistically significant. Overall comparisons between groups were performed using SPSS program (version 12.0, SPSS Inc., Chicago, IL, USA). Student's t-test and ANOVA followed by Tukey's test were needed to detect differences between groups in multiple comparisons.
To evaluate the effect of anthocyanin on the viability of human DU-145 cells, we used an MTT assay. Anthocyanin treatment significantly inhibited cell growth in a dose-dependent manner at all time points tested (24, 48, and 72 hours) (Fig. 1A). The IC50 value for anthocyanin at 24 hours after treating DU-145 cells was 60-90 µM. Whereas similar anthocyanin doses had insignificant effects on the viability of a normal prostate cell line, RWPE-1 (Fig. 1B). To test whether the anthocyanin-mediated decrease in cell growth was due to apoptosis, we carried out a DNA laddering assay to assess the fragmentation of cellular DNA, a characteristic of apoptotic cells. Agarose gel electrophoreses of the total DNA isolated from DU-145 cells treated with specified anthocyanin concentrations (60 or 120 µM for 24 or 48 hours) showed the characteristic DNA laddering patterns (Fig. 1C).
To elucidate the mechanism by which anthocyanin induces apoptosis in DU-145 cells, we next evaluated the effect of anthocyanin on the levels of apoptotic markers. Western blot analysis showed a significant increase in p53 expression in cells treated with anthocyanin (Fig. 2A and E). We also observed that anthocyanin treatment significant decreased the level of Bcl-2 protein with a concomitant increase in Bax protein (Fig. 2A, B, and C). These changes substantially increased the Bax/Bcl-2 ratio (Fig. 2D), which favors apoptosis, suggesting that the change in p53 and Bax/Bcl-2 ratio following anthocyanin treatment might trigger apoptosis.
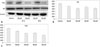
To determine the effects of anthocyanin on AR expression, a critical target for treating prostate cancer, and on PSA production, a western blot analysis was performed on DU-145 cells treated with specified anthocyanin concentration. AR and PSA were constitutively expressed in untreated DU-145 cells. However, PSA levels were considerably reduced in anthocyanin-treated cells compared to controls (Fig. 3A and B). Similar to PSA, AR expression was decreased in cells treated with anthocyanin (Fig. 3A and C).
The effect of anthocyanin on the cellular NAD+/NADH ratio in DU-145 cells was quantitatively assessed by ELISA. NAD+/NADH ratio in DU-145 cells were 1.684±0.406, 3.177±0.351, and 2.069±0.311 in the control, and treated with 60 or 120 µM for 24 or 48 hours, respectively. As shown in Fig. 4, NAD+/NADH ratio was genrally increased after anthocyanin treatment. A significant increase in NAD+/NADH ratio was found in the 120 µM-treated for 24 and 48 hours groups compared to the control group (p<0.05).
To study the inhibitory effects of anthocyanin on tumor growth in vivo, DU-145 tumor xenografts were established in athymic nude mice. The animals did not exhibit any considerable changes in body weight during the experiment. The in vivo anti-tumorigenic effects of anthocyanin were analyzed every 4 weeks for a total of 12 weeks. At 4 weeks post-inoculation, the initial detection time and average tumor volume did not differ significantly between the 2 groups. At 8 weeks postinoculation, however, the average tumor volume in vehicle-treated mice increased, reaching 317.3 mm3. At 8 weeks, the average tumor volume was only 119.5 mm3 in mice treated with anthocyanin. At 12 weeks post-inoculation, the average tumor volume in the control group increased to 831.3 mm3, while the anthocyanin group grew to 288.4 mm3 (Fig. 5). In addition, one mouse in the control group died. The differences in tumor development between anthocyanin- and vehicle-treated mice were statistically significant at 8 and 12 weeks post-inoculation (p<0.01) (Fig. 5).
Several reports8,13,14 suggest that oxidative stress is an early event in carcinogenesis and contributory risk factor for developing prostate cancer. Therefore, we used a powerful antioxidant agent, anthocyanin, and examined its effects on DU-145 prostate cancer cells and a xenograft tumor model.
The main findings of the present study were as follows: 1) anthocyanin treatment induced apoptosis of DU-145 cells through cell cycle arrest, which was accompanied by changes in p53, Bax, and Bcl-2 expressions with increased NAD+/ NADH ratio; 2) anthocyanin treatment decreased PSA and AR expressions in DU-145 cells; 3) anthocyanin treatment inhibited the growth of DU-145 xenografts in nude mice, confirming the inhibitory effects of anthocyanin on tumor growth in vivo. These changes in apoptosis and related factors in vitro are in accordance with the inhibitory effect of anthocyanin on the growth of xenograft tumor created by inoculation of DU-145 cells.
Therefore, anthocyanin may represent a promising agent for treating pathogeneses that result from oxidative stress. To further elucidate the mechanism and target molecules of anthocyanin, we evaluated the apoptosis pathways. Two important groups of proteins involved in apoptotic cell death are the Bcl-2 family members19 and caspases.20 The Bcl-2 family can be classified into 2 functionally distinct groups: Bcl-2, an anti-apoptotic protein, and Bax, a pro-apoptotic protein. Bcl-2 encodes a 26 kD protein that potently blocks apoptosis.21 Therefore, Bcl-2 affects neoplastic cell proliferation by preventing apoptosis.21,22 Bcl-2 is highly expressed in androgen-independent prostate cancer, but it is not strongly expressed in normal human prostate tissue.23,24 On the other hand, Bax promotes apoptosis:25 Bax heterodimerizes with Bcl-2 and neutralize its apoptotic effects. When Bax is overexpressed, apoptotic death signals increase. When Bcl-2 is overexpressed, it heterodimerizes with Bax, and death signals decrease. Thus, the ratio of Bax to Bcl-2 determines a cell's susceptibility to apoptosis.25,26 Indeed, previous studies showed that increased Bax expression or decreased Bcl-2 expression induced apoptosis in several prostate cancer cell lines, such as DU-145, PC-3, and LNCaP cells,27,28,29,30 suggesting that controlling of Bax and Bcl-2 expression is a promising approach for treating prostate cancers.
The Bax/Bcl-2 ratio can be altered by p53, "the guardian of the genome," which is a tumor suppressor protein that initiates apoptosis in cells with DNA damage.31,32 p53 might initiate apoptosis by directly altering the transcriptional activity of the Bax gene. This tumor suppressor binds to the Bax gene promoter and directly activates Bax transcription. Thus, p53 is an upstream regulator of Bax.
Our data showed that anthocyanin treatment of DU-145 cells 1) down-regulated Bcl-2 protein levels and 2) up-regulated Bax levels, which significantly increased the Bax/Bcl-2 ratio, with a concomitant increase in p53 expression. These apoptosis induction was accompanied with increased NAD+/NADH ratio, thus, antioxidant effect of anthocyanin could play a role in inducing apoptosis, suggesting that anthocyanin induced apoptosis of DU-145 cells by up-regulating p53, which modulated the Bax/Bcl-2 ratio. Recently, several studies reported that anthocyanin-induced apoptosis depends on upstream activation of MAPK and JNK pathways (caspase dependent and caspase-independent).33,34 Therefore, further studies are necessary to explore the involvement of apoptosis-related proteins and pathways after anthocyanin treatment.
Although androgen blockade treatment is effective for prostate cancer, it's antitumor effects can be temporary; the castration-sensitive phenotype will convert to a castration-resistant phenotype. Although defined as castration-resistant, androgen signaling through AR plays an important role in the development of androgen-resistant prostate cancer. This cancer may develop through constitutive AR activation (through gene amplification,35 alternative splicing,36 or an AR-activating gene mutation37), intratumoral androgen production, AR promiscuity, or downstream target activation by dysregulation of transcription factors.38 Reducing AR expression may represent an attractive approach to targeting androgen signaling in androgen-resistant prostate cancer. Recently, reduced AR signaling by the use of synthetic siRNA, AR antisense, geldanamycin analogs, and selective AR modulators, which demonstrated that downregulation On AR expression was sufficient to slow prostate tumor growth and induce apoptosis.39,40,41,42
In this study, we demonstrated that anthocyanin decreased AR expression along with inducing apoptosis in DU-145 cells in vitro. This in vitro effect correlated well with growth inhibition in vivo in a DU-145 xenograft model. A significant increase in the Bax/Bcl-2 ratio with a concomitant increase of p53 expression in anthocyanine-treated DU-145 cells is very similar to molecular mechanism involved in the xenograft model.
This study is the first to demonstrate that anthocyanin extracted from black soybean can inhibit and suppress prostate cancer progression in vitro and in a xenograft model. The initial and important step of prostate cancer development is DNA damage by oxidative stress. Therefore, we hypothesize that administering anthocyanin, which has a strong antioxidant effect, will inhibit prostate cancer progression. To go one step further to clinical application, future work should establish the relevance of in vitro findings to in vivo situation. In the present study, we observed that anthocyanin significantly reduced tumor growth of DU-145 cells xenograft. However, to examine the actual effect, we must confirm relevant effect in orthotopic prostate cancer model, in which the immune suppressed mice are implanted with prostate cancer cells directly into prostate. These in vivo growth inhibitory effects of anthocyanin should be correlated well with the induction of apoptosis and inhibition of known biomarkers (prostate specific antigen). In addition, toxicity tests are necessary to determine clinical usefulness.
Figures and Tables
Fig. 1
Effect of anthocyanin on the viability and apoptosis of DU-145 cells. (A) DU-145 cells were treated with specified anthocyanin concentrations at each treatment time, and cell viability was determined by MTT assay. (B) Viability of DU-145 cells in specified anthocyanin concentrations after 24 hr. (C) Results of a DNA laddering assay in DU-145 cells treated with specified anthocyanin concentrations.

Fig. 2
The effect of anthocyanin on p53, Bax, and Bcl-2 Expression in DU-145 cells. (A) Western blot analysis of p53, Bax, and Bcl-2. (B) Densitometric analysis of Bax relative to β-actin. (C) Densitometric analysis of Bcl-2 relative to β-actin. (D) The relative ratio of Bax to Bcl-2. (E) Densitometric analysis of p53 relative to β-actin.
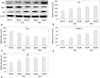
Fig. 3
The effect of anthocyanin on PSA and androgen receptor expression in DU-145 cells. (A) Western blot analysis of PSA and AR. (B) Densitometric analysis of PSA relative to β-actin. (C) Densitometric analysis of AR relative to β-actin. PSA, prostate specific antigen; AR, androgen receptor.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ009546), Rural Development Administration, Republic of Korea.
References
2. Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, et al. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat. 2009; 41:122–131.
3. Von Löw EC, Perabo FG, Siener R, Müller SC. Review. Facts and fiction of phytotherapy for prostate cancer: a critical assessment of preclinical and clinical data. In Vivo. 2007; 21:189–204.
4. Park SK, Sakoda LC, Kang D, Chokkalingam AP, Lee E, Shin HR, et al. Rising prostate cancer rates in South Korea. Prostate. 2006; 66:1285–1291.

5. Demark-Wahnefried W, Robertson CN, Walther PJ, Polascik TJ, Paulson DF, Vollmer RT. Pilot study to explore effects of low-fat, flaxseed-supplemented diet on proliferation of benign prostatic epithelium and prostate-specific antigen. Urology. 2004; 63:900–904.

7. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006; 160:1–40.

8. Bostwick DG, Alexander EE, Singh R, Shan A, Qian J, Santella RM, et al. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer. 2000; 89:123–134.

9. Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996; 273:59–63.

10. Pamplona R, Barja G. Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim Biophys Acta. 2006; 1757:496–508.

11. Warner HR. Superoxide dismutase, aging, and degenerative disease. Free Radic Biol Med. 1994; 17:249–258.
12. Mo JQ, Hom DG, Andersen JK. Decreases in protective enzymes correlates with increased oxidative damage in the aging mouse brain. Mech Ageing Dev. 1995; 81:73–82.
13. Malins DC, Johnson PM, Wheeler TM, Barker EA, Polissar NL, Vinson MA. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001; 61:6025–6028.
14. Malins DC, Johnson PM, Barker EA, Polissar NL, Wheeler TM, Anderson KM. Cancer-related changes in prostate DNA as men age and early identification of metastasis in primary prostate tumors. Proc Natl Acad Sci U S A. 2003; 100:5401–5406.

15. Jang H, Kim SJ, Yuk SM, Han DS, Ha US, Hong SH, et al. Effects of anthocyanin extracted from black soybean seed coat on spermatogenesis in a rat varicocele-induced model. Reprod Fertil Dev. 2012; 24:649–655.

16. Jang H, Ha US, Kim SJ, Yoon BI, Han DS, Yuk SM, et al. Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat model. J Agric Food Chem. 2010; 58:12686–12691.
17. Gong J, Traganos F, Darzynkiewicz Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem. 1994; 218:314–319.
18. Alessandri G, Filippeschi S, Sinibaldi P, Mornet F, Passera P, Spreafico F, et al. Influence of gangliosides on primary and metastatic neoplastic growth in human and murine cells. Cancer Res. 1987; 47:4243–4247.
19. Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999; 59:7 Suppl. 1693s–1700s.

21. Akao Y, Otsuki Y, Kataoka S, Ito Y, Tsujimoto Y. Multiple subcellular localization of bcl-2: detection in nuclear outer membrane, endoplasmic reticulum membrane, and mitochondrial membranes. Cancer Res. 1994; 54:2468–2471.
22. Danial NN. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin Cancer Res. 2007; 13:7254–7263.

23. Ohigashi T, Ueno M, Nonaka S, Nakanoma T, Furukawa Y, Deguchi N, et al. Tyrosine kinase inhibitors reduce bcl-2 expression and induce apoptosis in androgen-dependent cells. Am J Physiol Cell Physiol. 2000; 278:C66–C72.
24. Rosser CJ, Reyes AO, Vakar-Lopez F, Levy LB, Kuban DA, Hoover DC, et al. Bcl-2 is significantly overexpressed in localized radio-recurrent prostate carcinoma, compared with localized radio-naive prostate carcinoma. Int J Radiat Oncol Biol Phys. 2003; 56:1–6.

25. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993; 74:609–619.

26. Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996; 88:386–401.

27. Marcelli M, Marani M, Li X, Sturgis L, Haidacher SJ, Trial JA, et al. Heterogeneous apoptotic responses of prostate cancer cell lines identify an association between sensitivity to staurosporine-induced apoptosis, expression of Bcl-2 family members, and caspase activation. Prostate. 2000; 42:260–273.

28. Lowe SL, Rubinchik S, Honda T, McDonnell TJ, Dong JY, Norris JS. Prostate-specific expression of Bax delivered by an adenoviral vector induces apoptosis in LNCaP prostate cancer cells. Gene Ther. 2001; 8:1363–1371.

29. Chang HK, Shin MS, Yang HY, Lee JW, Kim YS, Lee MH, et al. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol Pharm Bull. 2006; 29:1597–1602.

30. Li X, Marani M, Yu J, Nan B, Roth JA, Kagawa S, et al. Adenovirus-mediated Bax overexpression for the induction of therapeutic apoptosis in prostate cancer. Cancer Res. 2001; 61:186–191.
31. Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995; 80:293–299.

32. Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, et al. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994; 9:1799–1805.
33. Reddivari L, Vanamala J, Chintharlapalli S, Safe SH, Miller JC Jr. Anthocyanin fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase-dependent and caspase-independent pathways. Carcinogenesis. 2007; 28:2227–2235.

34. Karna P, Gundala SR, Gupta MV, Shamsi SA, Pace RD, Yates C, et al. Polyphenol-rich sweet potato greens extract inhibits proliferation and induces apoptosis in prostate cancer cells in vitro and in vivo. Carcinogenesis. 2011; 32:1872–1880.

35. Linja MJ, Savinainen KJ, Saramäki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001; 61:3550–3555.
36. Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010; 120:2715–2730.

37. Tilley WD, Buchanan G, Hickey TE, Bentel JM. Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin Cancer Res. 1996; 2:277–285.
38. Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009; 15:4792–4798.

40. Singh P, Uzgare A, Litvinov I, Denmeade SR, Isaacs JT. Combinatorial androgen receptor targeted therapy for prostate cancer. Endocr Relat Cancer. 2006; 13:653–666.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download