Abstract
Purpose
Children with attention-deficit/hyperactivity disorder (ADHD) often perform poorly during cognitive tests. We sought to evaluate cortisol as potential moderator of performance in mentally challenging tasks in children with ADHD.
Materials and Methods
Ninety clinic-referred children with ADHD were studied. Cortisol contents in saliva were measured before and after administration of a continuous performance test (CPT).
Results
Pre and post CPT cortisol levels were similar in 68 children. Children whose cortisol level increased after testing ( n = 22 ) displayed a significantly longer response time and increased response time variability scores as compared to children who did not display increase of cortisol after the CPT test. Even after controlling for the effects of response time and anxiety, the changes in cortisol levels were associated with effect on response time variability.
Conclusion
The patients who showed an increased cortisol level after stress displayed a higher variability in response time than the patients who showed no change or a decreased cortisol level. The result of the current study suggests that stress-induced high norepinephrine (NE) release may accompany poorer attention performance in patients with ADHD.
Attention-deficit/hyperactivity disorder (ADHD) is a common neuropsychiatric disorder among children and adolescents.1 Up to 12% of children in the United States2 and 6.5-7.6% of children in Korea were reported to have ADHD.3,4 According to the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV), the core symptoms of ADHD consist of inattention, hyperactivity, and impulsivity.5 In addition to the core symptoms, associated learning problems, social deficits, and psychiatric comorbidity are predictive of a worse prognosis.6,7
Norepinephrine (NE) has a crucial role in the attention control of the frontal cortex.8-10 Activation of the post synaptic α2 adrenergic receptor is related to the engagement of attention,9 and α2 adrenergic agonists have been shown to have beneficial effects on working memory and attention performance, and reduce distractibility. However, a higher level of adrenergic activation seems to have a detrimental effect on attention performance via α1 adrenergic receptor activation.10 Since the α1 adrenergic receptor has a lower affinity for NE than the α2 adrenergic receptor, higher levels of NE are required to activate the α1 adrenergic receptor. However, increased levels of NE induced by stress impair attention control mediated by prefrontal cortical function.10-12
α2 adrenergic agonists have been used to control the symptoms of ADHD.9,13,14 In ADHD, adrenergic activation is also related to increased attention performance via α2 activation.8 Excessive adrenergic activation has been suggested to lead to α1 activation and impaired attention performance.9 However, few studies have directly examined the relationship between α1 activation and attention impairment. Increases of cortisol are associated with the stress-induced NE release and α1 adrenergic receptor activation.15,16 Moreover, increase of cortisol level after stress is mediated by the activation of the adrenergic system and α1 adrenergic receptors.15 Increased NE during stress activates α1 adrenergic receptors of the corticotrophin- releasing hormone (CRH) containing cells in the paraventricular nucleus of the hypothalamus, which leads to an increased cortisol level.17
Activation of α1 adrenergic receptor mediates both cortisol level increase and attention impairment. Based on these findings, we speculated that cortisol increase during mentally challenging tasks is related to poor attention performance. Therefore, we undertook this study to examine whether increased cortisol level after a mentally challenging task is associated with poor attentional performance in ADHD youth. Since the increased variability in response time is one of the most consistent findings in the neuropsychological research of children with ADHD,18-22 we focused our study on the response variability of attention performance.
Ninety clinic-referred children with ADHD participated in the study. A detailed protocol was described elsewhere, and data from a subsample were analyzed and published to address the relationship between stress reactivity and intellectual functioning.23 The participants were 6-15 years old and were mainly from the urban area of Seoul, Korea. ADHD was diagnosed using the Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL). We excluded children with comorbid psychiatric disorder, medical illness requiring medication, or with a prior history of taking ADHD medication. Written informed consent was obtained from the parents of the children after the purpose and process of the study were explained. The protocol of this study was approved by the Institutional Review Board (IRB) at Kangbuk Samsung Hospital.
In previous studies, psychological testing, Trier Social Stress Test, or routine dental examination were used as stressors to evaluate the hypothalamo-pituitary-adrenal (HPA) axis reactivity in ADHD patients.24-28 Psychological testing was used as a stressor to activate the HPA axis reactivity. In the present study, we adopted a psychological test including the Korean-Wechsler Intelligence Scale for Children-Third Edition (K-WISC-III) and ADHD Diagnostic System (ADS).29 The Korean version of Wechsler Intelligence Scale for Children-III (K-WISC-III)30 was standardized among Korean children and adolescents. The WISC-III was developed to assess intellectual functioning in children 6-16 years of age, and consists of 13 individual subsets, including 10 standard and 3 supplementary subsets. The verbal intelligence quotient (IQ) test consists of subsets pertaining to information, similarities, arithmetic, vocabulary, comprehension, and digit span, while performance IQ consists of subsets pertaining to picture completion, coding, picture arrangement, block design, object assembly, symbol search, and mazes. The test takes 50-70 minutes to finish all 10 standard subsets. The ADHD diagnostic system (ADS)29 is a computerized continuous performance test that consists of auditory and visual modalities. In each modality, the target and non-target are presented in the form of auditory or visual stimuli. The test can be used to assess children over five years of age and consists of three sessions: early, middle, and late. The variables include omission error, commission error, response time, and variability in response time, which reflects the standard deviation of response time.
All of the pre-test saliva samples were collected between 10 : 30 and 11 : 00 a.m. Participants were asked to take 30 minutes of rest before taking test. The patient's mouth was rinsed with water, and a saliva sample was taken 15 minutes before the WISC-III and ADS tests were performed. Thirty minutes after psychological testing, the patient's mouth was rinsed with water and a post-test saliva sample was collected.
The specimen was frozen at -20℃ to precipitate the mucin. The salivary cortisol concentration was determined by a standardized radioimmunoassay using the Diagnostic Product Corporation's Coat-A-Count Cortisol Kit (Los Angeles, CA, USA).
In order to evaluate the severity of ADHD symptoms, the Abbreviated Conners Rating scale (ACRS)31 was used. ACRS is a standardized measure of ADHD symptoms which is frequently used to evaluate response to treatment progress in ADHD youth.32 The Korean version of the ACRS has shown adequate reliability.31
Dimensional measures of behavioral problems were obtained with the Korean-Child and Adolescent Behavior Checklist (K-CBCL).33 The K-CBCL consists of 114 items, each rated on a three tier scale: 0 (never), 1 (sometimes), 2 (frequently/severe). The scores of the CBCL were standardized among Korean children and adolescents.
The State-Trait Anxiety Inventory for Children (STAIC)34 was used to evaluate anxiety severity. The state anxiety score reflects the current level of anxiety, and the trait anxiety score reflects severity of trait anxiety in the past 2 weeks. A Korean version of the STAIC was developed, and showed good reliability and validity among Korean children and adolescents.
Parents were asked to complete the rating scales within a week prior to the psychological testing.
The patients with ADHD were divided into two groups, based on cortisol level change after psychological testing. Group 1 consisted of the patients who did not show any increase in cortisol level after the psychological testing, while group 2 consisted of the patients who showed an increase of cortisol level after testing.
To test whether the increase in cortisol level was related to the greater variability in response time, analysis of covariance (ANCOVA) was performed. The variability score for ADS was entered as a dependent variable, and the group was entered as a fixed factor. Interaction between anxiety and sustained attention ability has been reported.35,36 Since anxiety may influence the cortisol change after a task,37 we controlled the effect of anxiety.
Since response time variability is highly correlated with response time,21 we controlled the effect of response time. To control the effect of anxiety and response time on the variability in response time, these variables were entered as covariates. Statistical Package for the Social Sciences (SPSS) 13.0 (SPSS Inc., Chicago, IL, USA) was used and statistical significance was set at p < 0.05.
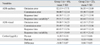
Sixty-eight out of ninety patients did not show an increase in cortisol level after psychological testing; these patients were designated as group 1. Children whose cortisol level increased were designated as group 2. Demographic data are presented in Table 1. There were no significant differences in age, gender, or scores of ACRS, CBCL, IQ test, and STAIC between group 1 and group 2 (Table 1).
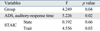
The ADS scores and cortisol levels are shown in Table 2. Group 2 revealed a significantly higher response time and response time variability values than group 1 (t = -2.655, df = 88, p = 0.009; t = -2.390, df = 88, p = 0.019, respectively).
After controlling the effect of response time and anxiety, there was a significant difference in response time variability between the groups (Table 3).
The children whose cortisol level increased after psychological testing displayed more variability in response time than children and adolescents who showed no change or a decreased cortisol level. Perhaps, α1 adrenergic activation in both the paraventricular nucleus (PVN) and prefrontal cortex (PFC) would explain such a result. Hypothalamopituitary-adrenal (HPA) axis reactivity and PFC response to stress are closely related.16,17 NE activates α1 adrenergic receptors of the PVN, which releases CRH.16 PFC also has CRH receptors, and modulates the HPA axis reactivity to stress.17 Increased intra-individual variability in speeded-reaction-time (RT) tasks has a significant predictive power to differentiate between patients with ADHD and normal controls.22,38 Increased variability in response time is related to poor attention control by the frontal cortex.18,20 The result of the current study suggests that high levels of NE released by stress may induce poor attention performance in ADHD patients.
Although the mechanism is quite complex, it is likely that methylphenidate improves attention in part via α2 adrenergic receptor activation. However, overdose of methylphenidate may result in sympathomimetic symptoms, including agitation.39 On the other hand, stress-induced adrenergic over-activation can lead to distraction.40 Local infusion of alpha-1 adrenergic agonist (phenylephrine) into the prefrontal cortex impaired the spatial working memory performance in monkeys.41 Arnsten, et al.9 suggested that patients who show a high level of distractibility may receive a beneficial effect from α1 adrenergic blocking agents. Further research regarding the effect of α1 adrenergic blocking agents on attention performance is necessary.
In the present study, the test result of only the auditory modality of ADS was associated with increased cortisol level. This is highly likely because the auditory modality is more difficult than the visual one, thus likely being more sensitive to detect attention impairment.29
Age may26,42 or may not43 affect the cortisol level. We concluded that age distribution did not distort the result of this study, since there was no significant difference in age distribution between group 1 and group 2.
There was no difference in anxiety between groups 1 and 2. The result of the regression analysis also showed that increase of cortisol level was related to an increased variability in response time after controlling the effect of anxiety.
Several limitations may require cautions when interpreting the result of the current study. We found an association between increased cortisol levels after stress and greater response variability in an ADHD sample. Since we did not have a control group, it is not clear whether the obtained findings are specific to ADHD. HPA axis reactivity to Trier Social Stress Test for Children (TSST-C) was significantly higher in girls than boys,44 and HPA axis reactivity was greater for men than for women in provocative virtual environment.28 These findings suggest that HPA axis responses may be modified by the interaction of gender and stress protocols. Few studies have been performed on the relationship between gender and stress reactivity to ADS. In our data, there was no significant difference in gender distribution between group 1 and group 2. Small sample size might cause negative finding. In the future, gender effect on the stress response to ADS needs to be examined with larger sample size. HPA axis reactivity is responsible for activating the glucocorticoid response to stress.45 However, a discrete set of information from brainstem aminergic/peptidergic afferents, bloodstream, limbic circuits and prefrontal cortex is also involved in HPA axis reactivity.46 In this study, we inferred α1 adrenergic activation from cortisol increase. As we did not directly measure and isolate α1 activity, we could not exclude the possibility that other factors might have also influenced the result. The attention performance in the prefrontal cortex is also regulated by other transmitters than noradrenalin.40,47 We did not take other neurotransmitters into consideration in this study. Consequently, we might have oversimplified the result. ADHD is a heterogenous neurodevelopmental disorder.1,47,48 In this study, only some of the patients with ADHD showed negative impact of stress on performance. Differential response of ADHD subtypes to stress has been suggested.49,50 The present result is likely to apply only to subgroup of patients with ADHD. Future study could aim at resolving subtype impact of stress on performance with larger sample size.
In spite of these limitations, the result of the current study suggests that stress-induced cortisol increase may be associated with poor attention performance in ADHD patients. The clinical implication of this study is that stress may worsen the attention performance and learning abilities of children with ADHD. According to our finding, children with ADHD who demonstrate significant change in cortisol are likely to function better in a less stressful educational environment. Further longitudinal research is needed to examine the relationship between ADHD and anxiety which may be exacerbated by stress, and to evaluate the potential to mediate response through stress reduction techniques such as relaxation, exercise or cognitive behavioral therapy.
Figures and Tables
Table 1
Dempographic and Descriptive Characteristics of the Samples Stratified by Changes in Cortisol Level with Decreased Post-Test Cortisol Levels (group 1) and Those with Increased Post-Test Cortisol Levels (group 2)

References
1. Pliszka S. AACAP Work Group on Quality Issuse. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007. 46:894–921.

2. Peterson BS, Pine DS, Cohen P, Brook JS. Prospective, longitudinal study of tic, obsessive-compulsive, and attention-deficit/hyperactivity disorders in an epidemiological sample. J Am Acad Child Adolesc Psychiatry. 2001. 40:685–695.
3. Yang SJ, Cheong S, Hong SD. Prevalence and correlates of attention deficit hyperactivity disorder: school-based mental health services in Seoul. J Korean Neuropsychiatr Assoc. 2006. 45:69–76.
4. Cho SC, Shin YO. Prevalence of disruptive behavior disorders. J Child Adolesc Psychiatry. 1994. 5:141–149.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). 1994. Washington DC: American Psychiatric Association.
6. Barkley RA. Barkley RA, editor. Associated Cognitive, Developmental, and Health Problems. Attention-Deficit Hyperactivity Disorder. A Handbook for Diagnosis and Treatment. 2006. 3rd ed. New York: The Guilford Press;123–183.
7. Barkley RA. Comorbid Disorders, Social and Family Adjustment, and Subtyping. Attention-Deficit Hyperactivity Disorder: a Handbook for Diagnosis and Treatment. 2006. 3rd ed. New York: The Guilford Press;184–218.
8. Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006. 67:Suppl 8. 7–12.
9. Arnsten AF, Scahill L, Findling RL. alpha2-Adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. J Child Adolesc Psychopharmacol. 2007. 17:393–406.

10. Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007. 113:523–536.
11. Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005. 28:403–450.

12. Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005. 493:99–110.

13. Chamberlain SR, Del Campo N, Dowson J, Müller U, Clark L, Robbins TW, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007. 62:977–984.

14. Kratochvil CJ, Wilens TE, Greenhill LL, Gao H, Baker KD, Feldman PD, et al. Effects of long-term atomoxetine treatment for young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006. 45:919–927.
15. al-Damluji S. Adrenergic mechanisms in the control of corticotrophin secretion. J Endocrinol. 1988. 119:5–14.

16. Plotsky PM, Cunningham ET Jr, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev. 1989. 10:437–458.

17. Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann N Y Acad Sci. 2004. 1018:25–34.
18. Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006. 10:117–123.

19. Hurks PP, Adam JJ, Hendriksen JG, Vles JS, Feron FJ, Kalff AC, et al. Controlled visuomotor preparation deficits in attention-deficit/hyperactivity disorder. Neuropsychology. 2005. 19:66–76.
20. Johnson KA, Kelly SP, Bellgrove MA, Barry E, Cox M, Gill M, et al. Response variability in attention deficit hyperactivity disorder: evidence for neuropsychological heterogeneity. Neuropsychologia. 2007. 45:630–638.

21. de Zeeuw P, Aarnoudse-Moens C, Bijlhout J, König C, Post Uiterweer A, Papanikolau A, et al. Inhibitory performance, response speed, intraindividual variability, and response accuracy in ADHD. J Am Acad Child Adolesc Psychiatry. 2008. 47:808–816.

22. Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005. 57:1416–1423.

23. Shin DW, Lee SH. Blunted hypothalamo-pituitary-adrenal axis reactivity is associated with the poor intelligence performance in children with attention-deficit/hyperactivity disorder. Neuropediatrics. 2007. 38:298–303.

24. Buske-Kirschbaum A, von Auer K, Krieger S, Weis S, Rauh W, Hellhammer D. Blunted cortisol responses to psychosocial stress in asthmatic children: a general feature of atopic disease? Psychosom Med. 2003. 65:806–810.

25. Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993. 28:76–81.

26. Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004. 29:83–98.

27. Blomqvist M, Holmberg K, Lindblad F, Fernell E, Ek U, Dahllöf G. Salivary cortisol levels and dental anxiety in children with attention deficit hyperactivity disorder. Eur J Oral Sci. 2007. 115:1–6.

28. Bullinger AH, Hemmeter UM, Stefani O, Angehrn I, Mueller-Spahn F, Bekiaris E, et al. Stimulation of cortisol during mental task performance in a provocative virtual environment. Appl Psychophysiol Biofeedback. 2005. 30:205–216.

29. Shin M, Cho S, Chun S, Hong K. A study of the development and standardization of ADHD diagnostic system. J Child Adolesc Psychiatry. 2000. 11:91–99.
30. Kim YS, Cheon KA, Kim BN, Chang SA, Yoo HJ, Kim JW, et al. The reliability and validity of Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version-Korean version (K-SADS-PL-K). Yonsei Med J. 2004. 45:81–89.

31. Oh KJ, Lee H. Assessment of ADHD with Abbreviated Conners Rating Scale. Korean J Clin Psychol. 1989. 8:135–142.
32. Green WH. Green WH, editor. General princilpes of psychopharmacotherapy with children and adolescents. Child and Adolescent clinical psychopharmacology. 1995. 2 ed. Baltimore: 7–46.
33. Oh K, Lee H, Hong K, Ha E. K-CBCL child and adolescent behavior checklist manual. 1997. Seoul: ChungAng Aptitude Pressing.
34. Cho SC, Choi JS. Development of the Korean form of the statetrait anxiety inventory for children. Seoul J Psychiatry. 1989. 14:150–157.
35. Ballard JC. Computerized assessment of sustained attention: interactive effects of task demand, noise, and anxiety. J Clin Exp Neuropsychol. 1996. 18:864–882.

36. Ballard JC. Assessing attention: comparison of response-inhibition and traditional continuous performance tests. J Clin Exp Neuropsychol. 2001. 23:331–350.

37. Granger DA, Weisz JR, Kauneckis D. Neuroendocrine reactivity, internalizing behavior problems, and control-related cognitions in clinic-referred children and adolescents. J Abnorm Psychol. 1994. 103:267–276.

38. Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intrasubject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006. 60:1088–1097.

40. Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999. 46:1266–1274.

41. Mao ZM, Arnsten AF, Li BM. Local infusion of an alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol Psychiatry. 1999. 46:1259–1265.

42. Kiess W, Meidert A, Dressendörfer RA, Schriever K, Kessler U, König A, et al. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995. 37:502–506.

43. Rosmalen JG, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC. Determinants of salivary cortisol levels in 10-12 year old children; a population-based study of individual differences. Psychoneuroendocrinology. 2005. 30:483–495.

44. Hatzinger M, Brand S, Perren S, von Wyl A, von Klitzing K, Holsboer-Trachsler E. Hypothalamic-pituitary-adrenocortical (HPA) activity in kindergarten children: importance of gender and associations with behavioral/emotional difficulties. J Psychiatr Res. 2007. 41:861–870.

45. Jankord R, Herman JP. Limbic regulation of hypothalamopituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008. 1148:64–73.

46. Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996. 10:371–394.

47. Faraone SV, Doyle AE. The nature and heritability of attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2001. 10:299–316. viii–viix.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download