Abstract
Purpose
Pseudoangiomatous stromal hyperplasia (PASH) of the breast is a rare, benign condition that can be mistaken as a fibroadenoma on an ultrasound examination or as a low-grade angiosarcoma on a histological examination. The objective of this study was to evaluate the ultrasound features and to present biopsy methods to correctly identify PASH.
Patients and Methods
We retrospectively reviewed the data of 55 women who were diagnosed with PASH of the breast. Ultrasound features were evaluated according to the Breast Imaging Reporting and Data System (BI-RADS; American College of Radiology). The diagnostic ability of different biopsy methods such as core needle biopsy, vacuum-assisted biopsy and excisional biopsy were analyzed with the final histopathological results of surgical specimens.
Results
PASH presented as a circumscribed solid mass, with hypoechoic texture with or without heterogeneity, and a parallel orientation. The features of small, internal cysts or vascular channels and no calcifications can be used to differentiate the lesions from fibroadenomas. A core needle biopsy misdiagnosed PASH in 13 cases out of 28 cases and vacuum-assisted biopsy correctly identified PASH in all 3 cases.
PASH of the breast was first described by Vuitch et al.1 in 1986. PASH presents as a benign stromal lesion containing complex anastomosing channels lined by slender spindle cells. In many cases, PASH is identified during a histological examination of tissue because of the presence of other tumors, but it presents as a painless, palpable, unilateral mass, ranging in size from 1 cm to 15 cm in approximately 40% of cases. Histologically, PASH can be mistaken for a low-grade angiosarcoma and phyllodes tumor, but it is a benign condition. Correct identification of PASH of the breast has important treatment implications for patients. To the best of our knowledge, few radiological characteristics differentiate this lesion from a fibroadenoma, but we were able to identify some imaging features that are specific to PASH. There have been several case reports of PASH;2-4 this study is a review of 55 cases of PASH, based mainly on ultrasound (US) findings. We present the results of the use of three biopsy techniques for making a histological diagnosis-core biopsy (with the use of a 14-gauge needle), vacuum-assisted biopsy (with the use of an 11-gauge or 8-gauge needle) and excision biopsy.
A retrospective review of the case records of 55 female patients with a final pathological diagnosis of PASH, diagnosed from March 2000 to March 2006 at Seoul Samsung Hospital or Kangbuk Samsung Hospital in Korea, was undertaken. Six of 55 patients were excluded from the study, because PASH was an incidental finding in mastectomy specimens in breast cancer without associated images. Three patients had no available ultrasound images. The age range for 49 patients ultimately enrolled in the study was 15 - 53 years, with a mean age of 41 years. All patients evaluated were premenopausal. Twenty-two of the study patients visited the clinic because of a palpable breast mass, and 27 patients were identified through screening breast US procedures.
We used an HDI 5000 (Advanced Technology Laboratories, Bothell, WA, USA), IU22 (Philips Medical Systems, Bothell, WA, USA) or LOGIQ 700 ultrasound scanner (GE Medical Systems, Milwaukee, WI, USA) equipped with a 12 - 5-MHz linear-array transducer. Experienced board-certified breast radiologists with at least 5 years of experience in breast US performed or supervised all sonographic examinations. We performed bilateral whole breast US including both axillae.
Pathologists who specialized in breast diseases made the initial diagnoses for the 49 patients in the study. Three diagnostic methods were employed: a 14-gauge needle core biopsy (28/49), an 8-guage or 11-guage needle vacuum-assisted biopsy (Mammotome, SCM 23, Ethicon, Endosurgery, Cincinnati, OH, USA) (9/49) depending on the tumor size and an excision biopsy (12/49). Indications for the biopsy were BI-RADS category 4a lesions or category 3 lesion with either palpable lesion or lesions with high risk history or when follow up US was not feasible. At least 5 specimens with a long diameter of 1 cm were obtained using a 14-guage needle and specimens from different areas of the lesion were obtained. For vacuum-assisted biopsies, specimens were obtained until the visualized tumor was totally removed under US guidance. For a nodule less than 2 cm in diameter an 11-guage needle was used and for larger nodules, an 8-guage needle was used. The specimens varied in size and number, and they were fixed in 10% formalin solution and were stained with haematoxylin-eosin. A pathologist reviewed all of the biopsy slides, and immunohistochemical studies were performed with anti-CD34, factor VIII-related antigen, and avidinbiotin-peroxidase complex to diagnose PASH.
Radiologists retrospectively analyzed the available US images of 46 patients. Image analysis was performed by 2 radiologists who correlated the final histopathology and US features originally described according to the Breast Imaging Reporting and Data System of the American College of Radiology.5 We correlated the pathological results according to the biopsy methods and the follow-up US findings.
Among 6 patients with benign-confirmed lesions who underwent further surgical excision, 1 was an image-pathology discordant case where the final pathology revealed stromal fibrosis with PASH. For the other 5 cases, palpable lesions causing pain or discomfort with triple assessment that showed no evidence of malignancy were candidates for surgical excision to promptly resolve the discomfort of patients.
Lesions ranged from 0.7 to 7 cm in size (mean size = 2.35 cm). Eighteen cases of PASH showed the presence of a well-circumscribed solid mass, with hypoechoic texture with or without heterogeneity, and parallel orientation (Fig. 1). The presence of small internal cysts or vascular channels was noted in 3 cases; these features were not found in fibroadenomas (Fig. 2). No calcifications were found, in contrast to findings for fibroadenomas.
BI-RADS categories of 46 masses were category 3 (probably benign) in 30 cases, category 4a (low suspicion of malignancy) in 15 cases (Fig. 3), and category 4b (intermediate suspicious of malignancy) in 1 case (Fig. 4). The nodule that was categorized as 4b was irregular in shape, not parallel to the breast axis with a spiculated margin, and was hypoechoic with posterior shadowing and an echogenic halo. This case was originally diagnosed as stromal fibrosis by a core biopsy, however the lesion was identified as PASH after an excisional biopsy. US findings of each nodule including shape, margin, internal echogenicities and posterior acoustic transmission are described in Table 1. The echogenicity of each lesion was compared with that of the surrounding normal fat lobules. One case showed an echogenic halo instead of an abrupt interface, and 2 lesions demonstrated internal vascular channels. Two cases of PASH were seen as a round mass that was not parallel to the chest wall (Fig. 5). Seven cases of non-mass forming, but depicted as only low echoic lesions, were intermingled with the normal parenchyma (Fig. 6).
Mammograms were available for 5 of 49 patients. Two patients showed oval, indistinct, high-density lesions with a background of extremely dense glandular parenchyme, and 3 patients showed asymmetrical densities. No characteristic radiological features of PASH were found in the mammograms.
PASH represented the initial target lesion in all but 6 specimens. The 6 cases with different diagnosis following a core biopsy were diagnosed as PASH after an additional excisional biopsy or vacuum-assisted biopsy.
Twenty-eight of 49 patients in our study underwent a core breast biopsy and 22 patients (78.6%) were diagnosed with PASH. PASH was not diagnosed by a core biopsy in 6 patients (21.4%); the patients were diagnosed with stromal fibrosis (4/28), fibrocystic changes (1/28) and a fibroadenoma (1/28). For these 6 patients, PASH was identified after an additional excisional biopsy (n = 5) or vacuum-assisted biopsy (n = 1). Among 28 patients who were diagnosed with PASH following an initial core biopsy, 16 patients underwent further surgical excision. A fibroadenoma was identified in 4 cases, predominant fibrocystic changes in 3 cases and one case of stromal fibrosis was identified. The final diagnosis for the other 8 patients was unchanged. Table 2 presents the initial pathology results according to the different biopsy methods employed and final pathology results for the patients who underwent further excision.
Nine of 49 patients were diagnosed with PASH following a vacuum-assisted biopsy. Of 9 patients, 3 patients underwent further excision, which confirmed the original diagnosis of 2 cases. An underestimated diagnosis of PASH combined with atypical lobular hyperplasia with low-grade carcinoma in situ was found in the third case, whereas the original diagnosis from an vacuum-assisted biopsy was PASH with intraductal papilloma (Table 2). The remaining 6 patients showed no residual lesion or recurrence on follow-up US.
In all cases, diagnosis included PASH (8/12), fibroadenoma, usual ductal hyperplasia and PASH (1/12), intraductal hyperplasia with focal atypia and PASH (1/12), stromal fibrosis with PASH (1/12), and fibrocystic changes, papillomatosis and PASH (1/12).
Follow-up imaging studies were available for 9 of 46 patients whose US studies were reviewed. Of 9 patients whose lesions were biopsied under US guidance, 2 patients had follow-up imaging that did not demonstrate any change in the lesion appearance. Follow-up imaging for 7 of 9 patients who underwent an excisional biopsy and 1 patient who underwent an vacuum-assisted biopsy with complete removal of the lesion demonstrated no evidence of recurrence. Follow-up studies were not available for the remaining patients.
PASH, a benign proliferation of stromal cells composed of myofibroblasts1 is associated with other benign entities, which include proliferative and nonproliferative fibrocystic changes, fibroadenomas, gynecomastia, and sclerosing lobular hyperplasia.6 It is found as an incidental finding in about 23% of breast biopsy specimens.7 Although these lesions do not occur commonly and they represent a diagnostic challenge, Piccoli et al.8 reported 13 cases of PASH out of 16 cases of biopsy specimen in developing asymmetric tissue after mammography, suggesting that PASH is a rather common histopathological finding in mammography with a growing asymmetric density. The pathological diagnosis of PASH may be difficult unless clinical information and radiological impressions are provided, so that the pathologic diagnosis can be focused more on stromal changes.
The etiology of mass-forming PASH is not known. Vuitch and colleagues1 and Rosen et al.9 have suggested that mass-forming PASH represents an exaggeration of normal physiological events that histologically resemble breast stromal cells in the luteal and secretory phases of the menstrual cycle.
The US appearances of PASH have previously been described by several investigators,2,4,8,10 but with limited number of cases. Polger et al.10 reported 3 of 4 masses as solid and hypoechoic, whereas 1 mass was heterogeneous with a small cystic component. Cohen et al.2 reported 6 of 7 lesions as solid, hypoechoic masses and 1 case of a spiculated hypoechoic nodule depicted on sonography. We identified several common sonographic characteristics of PASH with a large number of cases. Our study showed that not all of the lesions were oval, with the longitudinal axis of the lesion oriented parallel to the chest wall; 16 lesions showed an irregular shape, and 9 lesions were not parallel to the chest wall. Although most of the lesions identified in our study were solid and hypoechoic in echotexture, some lesions were heterogeneous in appearance. This heterogeneity may be due to inclusion of elements other than fibrous stroma (e.g., fibrocystic changes or adipose tissue). In 3 cases, the lesions demonstrated posterior acoustic shadowing. Sonography demonstrated the presence of vascular channels in 2 large lesions. In these cases, a histological examination also revealed the presence of vascular channels. On histopathology, the nodules that had peripheral echogenic rims consisted of a central area of PASH with irregular borders surrounded by adipose tissue with surrounding heterogenous parenchyma.
For tissue confirmation, several investigators have described cytological features of PASH, suggesting that the findings are non-specific.11-14 The differential diagnosis includes fibroadenoma and phyllodes tumor, fibrocytic change and low-grade sarcoma. Other investigators have reported false negative cases of PASH diagnosis after a core needle biopsy.15 When PASH is the predominant lesion determined after a needle core biopsy, correlation of clinical and ultrasound findings is critical to ensure that the targeted area was sampled. Our data indicate that an adequate target is not always obtained after a core biopsy, because the biopsied tissue sample is small and has heterogeneous histological composition of the lesion. In our study, 28 patients underwent a core biopsy, and 17/28 patients underwent follow-up excision or a vacuum-assisted biopsy. PASH was missed in 6 of these cases, and PASH was diagnosed only in the biopsy specimens in eight cases and was not observed in the specimens after further excision. Vacuum-assisted biopsy is not routinely used to remove PASH, however, a published report of vacuum-assisted resection of benign breast tumor16 presents the method as feasible and safe without serious complications as a single step, minimally invasive procedure. Based on our experience, a vacuum-assisted biopsy was also feasible and safe to practice, although it was not routinely applied for complete excision, but as a biopsy method when a specific and accurate diagnosis was required. Higher accuracy would be expected with the use of vacuum-assisted biopsy than the use of a core biopsy as the biopsied sample is larger.
The recommended treatment for PASH is wide local excision.17 A recently published study showed that non-surgical management strategies can be considered for patients who refuse a surgical procedure and options may be acceptable, especially when the lesion is small and triple assessment has been performed to exclude a malignancy.18 Some reports document an impressive response to tamoxifen in a patient presenting with breast enlargement, pain, and breast masses,19 however long term use may not be ideal, considering the potential side effects. None of the patients in our study was prescribed tamoxifen as a treatment drug.
The recurrence rates of PASH after the excision ranges from 15 to 22%,1,6,9,10 although we did not find any recurrence in the patients who underwent excision. However, complete follow-up evaluation was not performed for all of the patients, therefore a low recurrence rate is not conclusive.
A limitation of this study is that it was a retrospective study of PASH. The value of vacuum-assisted biopsy for PASH cannot be substantiated with only 3 cases that were surgically confirmed. Additional long-term follow-up studies are required to evaluate the outcome of vacuum assisted biopsy when further surgical excision was not undertaken. For patients who had surgical excision of PASH, longer follow-up studies are needed to evaluate the rate of recurrence. For patients who did not undergo surgical excision, more careful follow-up and clinical outcome should further be evaluated.
Best biopsy combination for inconclusive cases of PASH appears to be the combination of a core biopsy followed by an excisional biopsy. Radiologists should be informed about the US features of PASH; the lesions do not always present as an oval mass with parallel orientation. The diagnosis of PASH should carefully be considered by consultation with radiologist, pathologist and clinician.
Figures and Tables
Fig. 1
Transverse sonography of PASH shows an approximate 5 cm sized well-circumscribed homogeneous hypoechoic oval mass in a 30-year-old woman who presented with a palpable mass in the right breast. PASH, pseudoangiomatous stromal hyperplasia.
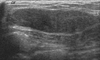
Fig. 2
(A) Sonography demonstrates a well-circumscribed homogeneous hypoechoic mass. (B) Doppler US shows vascular channels (arrows) in the lesion. In these cases, a histological examination also identified the presence of vascular channels. US, ultrasound.
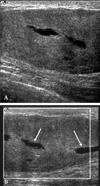
Fig. 3
Fibrocystic change was confirmed after an initial core needle biopsy, but surgical excision identified PASH in a 42-year-old premenopausal woman with BI-RADS category 4a. US shows a 2.8 cm sized heterogeneous echoic nodule mixed with an ill-defined margin with an irregular shape. PASH, pseudoangiomatous stromal hyperplasia; BI-RADS, breast imaging reporting and data system; US, ultrasound.
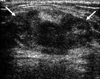
Fig. 4
Transverse (A) and longitudinal (B) breast US scans. A nodule, characterized as BI-RADS category 4b, was irregular in shape, not parallel to the breast axis with a spiculated margin, and was hypoechoic with posterior shadowing and an echogenic halo, which presented as a cancer or radial scar. This case was originally diagnosed after a core biopsy and was confirmed as stromal fibrosis, but an excisional biopsy identified the lesion as PASH. BI-RADS, breast imaging reporting and data system; PASH, pseudoangiomatous stromal hyperplasia; US, ultrasound.
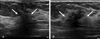
Fig. 5
Transverse (A) and longitudinal (B) breast US scans. This circumscribed round but not parallel to the chest wall PASH lesion was initially diagnosed as a fibroadenoma after a core biopsy. PASH, pseudoangiomatous stromal hyperplasia; US, ultrasound.
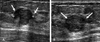
Fig. 6
Non-mass-forming PASH. PASH was depicted not as a mass but as low echoic lesions intermingled with the normal parenchyma. The initial pathological diagnosis of this case was fibrocystic change, but the lesion was identified as PASH after surgical excision. PASH, pseudoangiomatous stromal hyperplasia.
References
1. Vuitch MF, Rosen PP, Erlandson RA. Pseudoangiomatous hyperplasia of mammary stroma. Hum Pathol. 1986. 17:185–191.

2. Cohen MA, Morris EA, Rosen PP, Dershaw DD, Liberman L, Abramson AF. Pseudoangiomatous stromal hyperplasia: mammographic, sonographic, and clinical patterns. Radiology. 1996. 198:117–120.

3. Goel NB, Knight TE, Pandey S, Riddick-Young M, de Paredes ES, Trivedi A. Fibrous lesions of the breast: imaging-pathologic correlation. Radiographics. 2005. 25:1547–1559.

4. Mercado CL, Naidrich SA, Hamele-Bena D, Fineberg SA, Buchbinder SS. Pseudoangiomatous stromal hyperplasia of the breast: sonographic features with histopathologic correlation. Breast J. 2004. 10:427–432.

5. Mendelson EB, Baum JK, Berg WA, Merritt CR, Rubin E. . Ultrasound. Breast Imaging Reporting and Data System (BI-RADS). 2003. 4th ed. Reston, Va: American College of Radiology.
6. Powell CM, Cranor ML, Rosen PP. Pseudoangiomatous stromal hyperplasia (PASH). A mammary stromal tumor with myofibroblastic differentiation. Am J Surg Pathol. 1995. 19:270–277.
7. Brogi E. Benign and malignant spindle cell lesions of the breast. Semin Diagn Pathol. 2004. 21:57–64.

8. Piccoli CW, Feig SA, Palazzo JP. Developing asymmetric breast tissue. Radiology. 1999. 211:111–117.

9. Rosen PP. Rosen PP, editor. Benign mesenchymal neoplasms. Rosen's breast pathology. 1997. Philidelphia, PA: Lippincott Raven.
10. Polger MR, Denison CM, Lester S, Meyer JE. Pseudoangiomatous stromal hyperplasia: mammographic and sonographic appearances. AJR Am J Roentgenol. 1996. 166:349–352.

11. Aron M, Ray R, Verma K. Pseudoangiomatous stromal hyperplasia of the breast-cytological features of two cases and review of literature. Indian J Pathol Microbiol. 2005. 48:260–264.
12. Levine PH, Nimeh D, Guth AA, Cangiarella JF. Aspiration biopsy of nodular pseudoangiomatous stromal hyperplasia of the breast: clinicopathologic correlates in 10 cases. Diagn Cytopathol. 2005. 32:345–350.

13. Lui PC, Law BK, Chu WC, Pang LM, Tse GM. Fine-needle aspiration cytology of pseudoangiomatous stromal hyperplasia of the breast. Diagn Cytopathol. 2004. 30:353–355.

14. Spitz DJ, Reddy VB, Gattuso P. Fine-needle aspiration of pseudoangiomatous stromal hyperplasia of the breast. Diagn Cytopathol. 1999. 20:323–324.

15. Hoda SA, Rosen PP. Observations on the pathologic diagnosis of selected unusual lesions in needle core biopsies of breast. Breast J. 2004. 10:522–527.

16. Tagaya N, Nakagawa A, Ishikawa Y, Oyama T, Kubota K. Experience with ultrasonographically guided vacuum-assisted resection of benign breast tumors. Clin Radiol. 2008. 63:396–400.

17. Ibrahim RE, Sciotto CG, Weidner N. Pseudoangiomatous hyperplasia of mammary stroma. Some observations regarding its clinicopathologic spectrum. Cancer. 1989. 63:1154–1160.

18. Sng KK, Tan SM, Mancer JF, Tay KH. The contrasting presentation and management of pseudoangiomatous stromal hyperplasia of the breast. Singapore Med J. 2008. 49:e82–e85.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download