Abstract
Spinal angiomyolipoma (AML) is a rare disease. It is often reviewed with spinal angiolipoma. Both are composed of vascular and mature adipose elements. However, only AML contains broader array of mesenchymal component. They are accounting for 0.14% to 1.2% of spinal tumors. They appear as fat containing hypervascular tumor located at epidural space of thoracic spine. Spinal AML is more frequently infiltrative and ofted occurs more ventrally than angiolipoma. Previous studies have employed conventional radiograph, myelogram, and CT scan for spinal AML studies. Recently, MRI has been used for spinal AML in a few studies. Here, we describe a case of typical thoracic spinal AML with a review of its MRI findings and other differential diagnosis for epidural spinal mass with similar characteristics.
Angiomyolipoma (AML) is a benign neoplasm composed of vascular and mature adipose elements with broader array of mesenchymal components (1). Most AML tumors are found at the kidney (2). Extrarenal AML including spinal AML is rare. Compared to spinal AML, spinal angiolipoma contains both vascular and adipose elements without smooth muscle components. Spinal AML and spinal angiolipoma are always reviewed together because of their pathological and radiological similarities. They are accounting for 0.14% to 1.2% of spinal tumors (3).
Spinal AML and angiolipoma present as fat-containing hypervascular tumors located at the epidural space. The most common location is thoracic spine. However, spinal AML tends to be more frequently infiltrative It is generally located more ventrally than spinal angiolipoma (4).
These features are based on the radiologic modality of conventional radiograph and CT scan. A few studies on AML have used MRI (4). Here, we describe a case of thoracic spinal AML with typical characteristics. In addition, a review of MRI findings of spinal AML and other differential diagnosis for epidural spinal mass with similar radiologic findings is presented.
34-year-old woman visited our hospital for tingling sensation
of both legs which had lasted for 6 weeks and worsened with progressive weakness of both legs in the past week. Her tingling sensation involved entire surface of both legs without asymmetry. There was no back pain or radiating pain. Two weeks ago, she had a vaginal delivery for her second baby. She had developed mild but similar symptoms when she had delivered her first child two years ago. At that time, she had undergone conservative management with alternative therapy that improved her symptoms. She had no other significant medical or trauma history.
Her physical examination showed general weakness that was similar for both legs with decreased flexion function in all joints of both legs and relatively spared extension function. She could not flex her knee or ankle by herself. Both legs were hypoesthetic below the L1 level. No other pathologic reflex was noted except for bulbocarvenosus reflex.
Conventional radiographs of the thoracolumbar spine were grossly normal. No demonstrable vertebral scalloping or neural foraminal enlargement was noted. CT revealed an elliptical shape isodense mass lesion in posterior epidural space across five vertebral body segments between T6 and T9 (Fig. 1A). This lesion was compressing the dural sac anteriorly. Fat density was observed at the central portion and both the cephalic and caudal ends of the mass (Fig. 1A). No adjacent bony erosion or invasion was noted.
Both T1- and T2-weighted sagittal MRI revealed a 3.3 × 1.2 × 9.2 cm sized elliptical shape mass posteriorly located along the dural sac from T6 to T10, circumferentially compressing the spinal cord (Fig. 1B, C). The flattened and anteriorly displaced spinal cord showed no abnormal signal change, including myelopathy. Both sides of the neural foramen at T7–8 and T8–9 were slightly extended by the tumor. They were not associated with adjacent bone erosion (Fig. 1E). The mass was grossly heterogenous with intermediate signal intensity on both T1- and T2- weighted images. Mottled high-signal intensity area on T1-weighted image within the central portion and both the cephalic and caudal ends of the mass was suppressed on short tau inversion recovery image, suggesting fat component. Serpentine low-signal intensity on T2-weighted image mainly within the central portion of the mass was remarkable, probably resulting from vascular structure with fast flow (Fig. 1C). After gadolinium injection, the mass showed heterogenous and intense enhancement with central heterogenous signal intensity (Fig. 1D, E).
Total bilateral laminectomy on T7 and T8 and partial laminectomy on T6 and T9 with mass removal were performed. An elongated-shape lipomatous tumor with numerous vasculatures was found at the posterior epidural space.
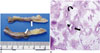
On pathologic examination, an elongated yellow gray solid mass measured 7 × 1.5 × 0.4 cm was noted. Its external surface was irregular. On section, the cut surface revealed bright yellow homogenous appearance and tortuous dilated vascular structures (Fig. 2A). Microscopically, the lesion was composed of three components: tortuous, thick-walled blood vessels, irregularly arranged sheets or perivascular bundles of myoid-appearing spindle cells, and lipid-distended mature adipocytes (Fig. 2B). The diagnosis of AML was confirmed.
After surgery, the patient's neurologic symptoms were improved with rehabilitative therapy. The patient walked with the aid of a cane at discharge. After 3 months, she could walk without assistance. Her hypoesthesia was also improved without any complications.
AML is a benign neoplasm composed of vascular and mature adipose elements with broader array of mesenchymal components (1). AMLs generally occur in the kidney. They are often associated with tuberous sclerosis (2). In contrast to renal AMLs, extrarenal AMLs are uncommon, with the liver and retroperitoneum being the most frequently involved (5). Spinal AML is very rare. Up to date, only a few cases have been reported in the literatures, including spinal angiolipoma that accounts for 0.14% to 1.2% of spinal tumors and 16% to 35% of spinal lipomas (excluding cases of myelovertebral anomalies) (3).
Histologically, a number of cases have been serially reviewed after spinal lipoma and angiolipoma were considered as distinct pathological entities (1). Spinal angiolipoma and spinal AML are linked together because of their pathological and radiological similarities. Both are composed of adipose and vascular elements. However, only AML contains smooth muscle component. Spinal AML was first described in a scientific journal in 1970 when Pearson et al. (6) reported two spinal AMLs appearing as variable angioid components with smooth muscle proliferation involving the media of numerous blood vessels and close association of osteoid tissue planes with tumor stroma (16). A few cases sharing these pathological characteristics have been reported after that (14).
The pathogenesis of AML and angiolipoma remains unclear. Two major theories have been previously proposed regarding the pathogenesis (14). One is that primitive pluripotential mesenchyme cells (which provides a common heritage to adipose, smooth muscle, and vascular endothelial elements) are developed into tumor by ill-defined stimuli (trauma or other causes). Another theory is that the tumor is a congenital malformation or true hamartoma.
Most patients with spinal AML and angiolipoma have complaints of neurological symptoms related to spinal cord compression, including weakness of the extremities and abnormal sensation below the level of lesion (7). Interestingly, the relationship between pregnancy and accelerated onset of neurologic symptoms has been reported in several cases of epidural spinal angiolipomas (1).
For radiological assessment of spinal AML and angiolipoma, previous studies have used conventional radiograph, myelography, or CT for the purpose of preoperative diagnosis (16). A recent few cases have employed MRI (4). Commonly, both spinal AML and angiolipoma appear as elliptical shaped soft tissue mass with heterogenous marked enhancement at epidural space of thoracic spine (123456). Fat component with high signal intensity on T1-weighted image is often visualized in cases of spinal angiolipoma (37). However, infiltrative properties such as invasion to adjacent bone or extension to perilesional space and the masses prone to ventral location have been mentioned as distinctive features of AML compared to major patterns of angiolipoma according to Sakaida et al. (4).
Our case included all the characteristics of spinal AML mentioned above. However, two different points were revealed. First, the lesion was located at dorsal aspect whereas most spinal AMLs were located at ventral aspect. Second, unusual serpentine signal voids on T2-weighted images were found. Most angiolipomas and AMLs are composed of capillaries and venous channels revealed as inhomogenous enhancement that is different from mass with fast-flowing arteriolar circulation such as arteriovenous shunting (2). However, our case showed unusual flow-void phenomenon that was also demonstrated on pathologic specimen as small arteriolar structures within the mass. This is an unusual and a unique point of our case.
Differential diagnosis of AML and angiolipoma includes fat-containing tumor (such as lipoma, lipomatosis, and liposarcoma), prominently vascular tumor (such as spinal epidural hemangioma), and other T1-high signal intensity lesion (such as epidural hematoma). Epidural lipoma or lipomatosis is an abnormal accumulation of unencapsulated adipose tissue in the extradural space (8). Because of their fat components, they also display hyperintensity on T1-weighted images and intermediate intensity on T2-weighted images. However, they present with typical Y configuration (with circumferentially compressed dural sac) and show no definite contrast enhancement pattern (28). Well-differentiated liposarcoma is a rare fat-containing tumor of the spinal canal. It frequently has irregular thick-ended septa. It contains regions of hyperintense signal (compared to fat) on T2-weighted images which are rare in spinal AML (2). Cavernous hemangioma is the most common type of epidural hemangioma. It frequently appears as lobulated-contour solid hypervascular mass of the spine similar to spinal AML (9). However, spinal epidural hemangioma could be differentiated from spinal AML due to its T2 low signal intensity rim (considered as fibrous capsule or hemosiderin deposition), multi-segment involvement, and rare T1-high signal intensity foci (except combined internal hemorrhage) (9). Subacute stage epidural hematoma can mimick spinal AML because of its heterogenous high signal intensity on T1-weighted images. However, fat suppression sequence can be used to distinguish hemorrhage from true fat component.
In summary, we report a case of spinal AML involving thoracic epidural space. This rare tumor shows relatively typical findings on MRI. It should be included as a differential diagnosis of spinal epidural tumor with fat component and high degree of vascularization.
Figures and Tables
Fig. 1
Spinal angiomyolipoma of T-spine in a 34-year-old woman.
A. Sagittal CT scan showing an elliptical shaped posterior epidural mass involving T6 to T10. Central portion and both cephalic and caudal ends of the mass have fat density (arrows).
B. Sagittal T1-weighted image showing the epidural mass circumferentially compressing the spinal cord.
C. Sagittal T2-weighted image showing serpentine or mottled low-signal intensity within central portion of the mass (arrow).
D. Sagittal T1 Gd-enhanced image showing relatively heterogenous enhancement with central serpentine or motteled non-enhanced foci.
E. Axial T1 Gd-enhanced image at T7–8 level showing both neural foraminal extension of the mass with central heterogenous signal intensity without adjacent bony erosion. Spinal cord is flattened ventrally displaced by the tumor.
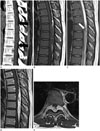
Fig. 2
Pathology of spinal angiomyolipoma of T-spine in a 34-year-old woman.
A. Gross specimen showing an elongated, yellow and gray solid mass with dilated and thrombosed vascular structures on cut surface (arrows). B. Microscopically, the lesion is composed of three components: tortuous thick-walled blood vessels (short arrow), irregularly arranged sheets or perivascular bundles of myoid-appearing spindle cells (long arrow), and lipid-distended mature adipocytes (curved arrow) (hematoxylin and eosin stain, × 40).
References
1. von Hanwehr R, Apuzzo ML, Ahmadi J, Chandrasoma P. Thoracic spinal angiomyolipoma: case report and literature review. Neurosurgery. 1985; 16:406–411.
2. Provenzale JM, McLendon RE. Spinal angiolipomas: MR features. AJNR Am J Neuroradiol. 1996; 17:713–719.
3. Preul MC, Leblanc R, Tampieri D, Robitaille Y, Pokrupa R. Spinal angiolipomas. Report of three cases. J Neurosurg. 1993; 78:280–286.
4. Sakaida H, Waga S, Kojima T, Kubo Y, Matsubara T, Yamamoto J. Thoracic spinal angiomyolipoma with extracanal extension to the thoracic cavity. A case report. Spine (Phila Pa 1976). 1998; 23:391–394.
5. Goldblum JR, Weiss SW, Folpe AL. Enzinger and Weiss's soft tissue tumors. 6th ed. Philadelphia: Elsevier Saunders;2013. p. 897–899.
6. Pearson J, Stellar S, Feigin I. Angiomyolipoma: long-term cure following radical approach to malignant-appearing benign intraspinal tumor. Report of three cases. J Neurosurg. 1970; 33:466–470.
7. Hu S, Hu CH, Hu XY, Wang XM, Dai H, Fang XM, et al. MRI features of spinal epidural angiolipomas. Korean J Radiol. 2013; 14:810–817.
8. Geers C, Lecouvet FE, Behets C, Malghem J, Cosnard G, Lengelé BG. Polygonal deformation of the dural sac in lumbar epidural lipomatosis: anatomic explanation by the presence of meningovertebral ligaments. AJNR Am J Neuroradiol. 2003; 24:1276–1282.
9. Lee JW, Cho EY, Hong SH, Chung HW, Kim JH, Chang KH, et al. Spinal epidural hemangiomas: various types of MR imaging features with histopathologic correlation. AJNR Am J Neuroradiol. 2007; 28:1242–1248.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download