INTRODUCTION
Undifferentiated embryonal sarcoma of the liver (UES) is a rare and highly malignant hepatic neoplasm of mesenchymal origin that occurs predominantly in children, usually those 6-10 years old (1). Adult UES are extremely rare, especially in the over 60 years (2). To our knowledge, no prior study has clarified how fast a tumor mass grows, even though it is known as a rapid-growing tumor. We report the unique case of adult UES that provide verification of the high growth rate of the tumor.
CASE REPORT
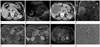
A 64-year female was admitted to emergency room of our hospital for acute right upper quadrant abdominal pain. Physical examination revealed a tender mass in the right upper quadrant of abdomen without fever. Laboratory data of liver function, tumor marker such as carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 125, CA19-9, and alpha-fetoprotein (AFP) were within normal range, except leukocytosis (white blood cell 14.070/uL, neutrophils 80.5%). He had a history of colon cancer (TxN0M0 by CT staging) with curative endoscopic mucosal resection 4 years ago. There had been no tumor recurrence during the follow-up period of about 4 years by abdominal CT. On the last follow-up CT before visiting to emergency room, there was a 0.4 cm hypodense lesion in segment 5 of liver (Fig. 1A). At that time, it was presumed to be a hepatic cyst.
Initial abdominal ultrasonography (US) showed the huge mass that was predominantly solid, heterogeneous in echogenicity, and hypovascular with cystic component in the periphery (Fig. 1B). Abdominal CT showed a 11.0 cm-sized, well-circumscribed hypoattenuating mass in the right lobe of the liver. On iodinated contrast enhanced CT, this cystic was compartmentalized by septations and showed no solid portion or calcification. Hemoperitoneum was seen mainly in the perihepatic space (Fig. 1C).
T2-weighted MR images showed a well-circumscribed large mass which was markedly hyperintense with multiple thick septa and cavities of variable sizes in the right lobe of liver as well. Coronal T2-weighted MR images showed clearly the ruptured portion of the tumor with intraperitoneal fluid (Fig. 1D). T1-weighted MR images showed the mass was predominantly hypointense with inner the foci of high signal intensity, representing hemorrhage (Fig. 1E). Gadolinium contrast enhanced T1-weighted MR images showed heterogeneous enhancement of thick septa and the periphery in the mass (Fig. 1F). The dense peripheral rim was hypointense on T1- and T2-weighted MR images corresponding to the fibrous pseudocapsule. Therefore, initial diagnosis was ruptured cystic tumor such as cystadenoma or cystadenocarcinoma.
Positron emission tomography-CT showed diffuse mild fluorodeoxyglucose uptake in the peripheral portion of the mass, but not in the inner portion of the mass (Fig. 1G). On US-guided biopsy, pathologic finding was not distinct. Consequently, surgical resection was considered.
Because of aggravated abdominal pain and increased size of the tumor on the follow-up CT, persistent bleeding from the cystic tumor was suspected. Angiography showed hypovascular mass with abnormal feeding vessels inside a tumor, and then gel-form embolization and right hepatic lobectomy were performed successfully. The solitary, large cystic mass of the liver was confirmed as primary UES (Fig. 1H).
During the retrospective evaluation of CT scans, we concluded that the hypodense lesion was early UES in that the center of UES corresponds with the location of it. UES had grown from 0.4 cm to 11.0 cm in a maximal diameter for 7 months.
DISCUSSION
UES is a rare, highly malignant neoplasm of the liver predominantly in children (1). UES in the old, especially over 40 s is extremely rare (2).
Patients frequently present with abdominal pain, fever, and weight loss. According to the literature as our case, laboratory findings of liver function and tumor marker such as AFP, CEA and CA19-9 are normal in most cases (2).
The UES has unique characteristic radiologic finding as in our case, that is a huge mass predominantly solid appearance on US, but cystic appearance on CT and MRI due to the high water content of the prominent myxoid stroma (3).
At US, UES appears iso- to hyperechoic, mainly solid mass correlates well with the pathologic findings (3, 4). The anechoic, cystic lesions correlate with cystic degeneration, old hemorrhage and necrosis (3).
The CT shows typically predominantly cystic mass as water attenuation with septa of variable thickness (3). The large portion of the mass is water-attenuation correlates with myxoid stroma of the pathologic findings (3-5). Occasionally, a pseudocapsule may be observed by a thick and enhancing peripheral rim (4). Acute hemorrhage may be also observed by central foci of high attenuation with fluid-debris levels (3). Internal calcifications are uncommon (4). Predominantly peripheral enhancement, especially on delayed images, have been reported after intravenous administration of iodinated contrast materials (3).
MR imaging characteristics also resemble those of cystic lesion as same as CT. Therefore, large portions of the mass are hypointense on T1-weighted images and hyperintense on T2-weighted images. Some high signal intensity on T1- and T2-weighted images represents intratumoral hemorrhage, which are better seen with MR imaging than CT (3, 4, 6). A hypointense on T1- and T2-image represents also the fibrous pseudocapsule (4). Heterogeneous enhancement of the peripheral and solid portions of the mass have been reported after intravenous administration of gadolinium contrast materials (7).
Owing to its cystic appearance at CT and MR imaging, sometimes UES can be mistaken for cystic tumor (8). Especially UES in the old or with presenting by rupture, initial correct diagnosis is not easy as our case.
Until recently, the prognosis of UES was poor, with mean survival of 12 months after diagnosis, even though complete surgical resection (1). But recent authors report use of multiagent, adjuvant, or neoadjuvant chemotherapy followed by resection offer the best long-term results and possibly a cure (9).
UES was known high malignant tumor and the fact that the tumor grows fast have been mentioned in several reports (2). However, it has not been supported by strong evidence in any other studies. In our case, during a colon cancer follow-up, several abdominal CT scans had been taken regularly. We could notice that the maximal diameter was increased from 0.4 cm to 11.0 cm just in 7 months on CT. As our knowledge, this is a first and valuable report in the way that it is possible to express the growth rate numerically.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download