1. Ohlson LO, Larsson B, Björntorp P, Eriksson H, Svärdsudd K, Welin L, Tibblin G, Wilhelmsen L. Risk factors for type 2 (non-insulin-dependent) diabetes mellitus: thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia. 1988. 31:798–805.
2. Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991. 338:774–778.
3. Kim DJ, Cho NH, Noh JH, Kim HJ, Choi YH, Jung JH, Min YK, Lee MS, Lee MK, Kim KW. Fasting plasma glucose cutoff value for the prediction of future diabetes development: a study of Middle-Aged Koreans in a Health Promotion Center. J Korean Med Sci. 2005. 20:562–565.
4. Fradin D, Heath S, Lathrop M, Bougnères P. Quantitative trait loci for fasting glucose in young Europeans replicate previous findings for type 2 diabetes in 2q23-24 and other locations. Diabetes. 2007. 56:1742–1745.
5. Huang QY, Cheng MR, Ji SL. Linkage and association studies of the susceptibility genes for type 2 diabetes. Yi Chuan Xue Bao. 2006. 33:573–589.
6. Frayling TM. Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet. 2007. 8:657–662.
7. Martin N, Boomsma D, Machin G. A twin-pronged attack on complex traits. Nat Genet. 1997. 17:387–392.
8. Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin: rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002. 30:97–101.
9. Sung J, Lee K, Song YM. Heritabilities of the metabolic syndrome phenotypes and related factors in Korean twins. J Clin Endocrinol Metab. 2009. 94:4946–4952.
10. Sham PC, Purcell S, Cherny SS, Abecasis GR. Powerful regression-based quantitative-trait linkage analysis of general pedigrees. Am J Hum Genet. 2002. 71:238–253.
11. Elston RC. On fisher's method of combining p-values. Biom J. 1991. 33:339–345.
12. Meigs JB, Manning AK, Fox CS, Florez JC, Liu C, Cupples LA, Dupuis J. Genome-wide association with diabetes-related traits in the Framingham Heart Study. BMC Med Genet. 2007. 8:S16.
13. Busfield F, Duffy DL, Kesting JB, Walker SM, Lovelock PK, Good D, Tate H, Watego D, Marczak M, Hayman N, et al. A genomewide search for type 2 diabetes-susceptibility genes in indigenous Australians. Am J Hum Genet. 2002. 70:349–357.
14. Hsueh WC, St Jean PL, Mitchell BD, Pollin TI, Knowler WC, Ehm MG, Bell CJ, Sakul H, Wagner MJ, Burns DK, et al. Genome-wide and fine-mapping linkage studies of type 2 diabetes and glucose traits in the Old Order Amish: evidence for a new diabetes locus on chromosome 14q11 and confirmation of a locus on chromosome 1q21-q24. Diabetes. 2003. 52:550–557.
15. Lindgren CM, Mahtani MM, Widén E, McCarthy MI, Daly MJ, Kirby A, Reeve MP, Kruglyak L, Parker A, Meyer J, et al. Genomewide search for type 2 diabetes mellitus susceptibility loci in Finnish families: the Botnia Study. Am J Hum Genet. 2002. 70:509–516.
16. Vionnet N, Hani EH, Dupont S, Gallina S, Francke S, Dotte S, De Matos F, Durand E, Leprêtre F, Lecoeur C, et al. Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. Am J Hum Genet. 2000. 67:1470–1480.
17. Ghosh S, Watanabe RM, Hauser ER, Valle T, Magnuson VL, Erdos MR, Langefeld CD, Balow J Jr, Ally DS, Kohtamaki K, et al. Type 2 diabetes: evidence for linkage on chromosome 20 in 716 Finnish affected sib pairs. Proc Natl Acad Sci U S A. 1999. 96:2198–2203.
18. Ghosh S, Watanabe RM, Valle TT, Hauser ER, Magnuson VL, Langefeld CD, Ally DS, Mohlke KL, Silander K, Kohtamäki K, et al. The Finland-United States investigation of non-insulin-dependent diabetes mellitus genetics (FUSION) Study. I: an autosomal genome scan for genes that predispose to type 2 diabetes. Am J Hum Genet. 2000. 67:1174–1185.
19. Tsai FJ, Yang CF, Chen CC, Chuang LM, Lu CH, Chang CT, Wang TY, Chen RH, Shiu CF, Liu YM, et al. A genome-wide association study identifies susceptibility variants for type 2 diabetes in Han Chinese. PLoS Genet. 2010. 6:e1000847.
20. Hanson RL, Bogardus C, Duggan D, Kobes S, Knowlton M, Infante AM, Marovich L, Benitez D, Baier LJ, Knowler WC. A search for variants associated with young-onset type 2 diabetes in American Indians in a 100K genotyping array. Diabetes. 2007. 56:3045–3052.
21. Kapp K, Metzinger E, Kellerer M, Häring HU, Lammers R. The protein tyrosine phosphatase alpha modifies insulin secretion in INS-1E cells. Biochem Biophys Res Commun. 2003. 311:361–364.
22. Ahmad F, Azevedo JL, Cortright R, Dohm GL, Goldstein BJ. Alterations in skeletal muscle protein-tyrosine phosphatase activity and expression in insulin-resistant human obesity and diabetes. J Clin Invest. 1997. 100:449–458.
23. Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995. 80:225–236.
24. Ostman A, Hellberg C, Böhmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006. 6:307–320.
25. Zheng X, Resnick RJ, Shalloway D. Apoptosis of estrogen-receptor negative breast cancer and colon cancer cell lines by PTP alpha and src RNAi. Int J Cancer. 2008. 122:1999–2007.
26. Wu CW, Kao HL, Li AF, Chi CW, Lin WC. Protein tyrosine-phosphatase expression profiling in gastric cancer tissues. Cancer Lett. 2006. 242:95–103.
27. Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996. 272:728–731.
28. Bossenmaier B, Mosthaf L, Mischak H, Ullrich A, Häring HU. Protein kinase C isoforms beta 1 and beta 2 inhibit the tyrosine kinase activity of the insulin receptor. Diabetologia. 1997. 40:863–866.
29. Standaert ML, Bandyopadhyay G, Galloway L, Soto J, Ono Y, Kikkawa U, Farese RV, Leitges M. Effects of knockout of the protein kinase C beta gene on glucose transport and glucose homeostasis. Endocrinology. 1999. 140:4470–4477.
30. Kaneto H, Suzuma K, Sharma A, Bonner-Weir S, King GL, Weir GC. Involvement of protein kinase C beta 2 in c-myc induction by high glucose in pancreatic beta-cells. J Biol Chem. 2002. 277:3680–3685.
31. Kawano Y, Rincon J, Soler A, Ryder JW, Nolte LA, Zierath JR, Wallberg-Henriksson H. Changes in glucose transport and protein kinase Cbeta(2) in rat skeletal muscle induced by hyperglycaemia. Diabetologia. 1999. 42:1071–1079.
32. Osterhoff MA, Heuer S, Pfeiffer M, Tasic J, Kaiser S, Isken F, Spranger J, Weickert MO, Möhlig M, Pfeiffer AF. Identification of a functional protein kinase Cbeta promoter polymorphism in humans related to insulin resistance. Mol Genet Metab. 2008. 93:210–215.
33. Wang Q, Lin JL, Reinking BE, Feng HZ, Chan FC, Lin CI, Jin JP, Gustafson-Wagner EA, Scholz TD, Yang B, et al. Essential roles of an intercalated disc protein, mXinbeta, in postnatal heart growth and survival. Circ Res. 2010. 106:1468–1478.
34. Takeuchi F, Katsuya T, Chakrewarthy S, Yamamoto K, Fujioka A, Serizawa M, Fujisawa T, Nakashima E, Ohnaka K, Ikegami H, et al. Common variants at the GCK, GCKR, G6PC2-ABCB11 and MTNR1B loci are associated with fasting glucose in two Asian populations. Diabetologia. 2010. 53:299–308.
35. Rasmussen-Torvik LJ, Alonso A, Li M, Kao W, Köttgen A, Yan Y, Couper D, Boerwinkle E, Bielinski SJ, Pankow JS. Impact of repeated measures and sample selection on genome-wide association studies of fasting glucose. Genet Epidemiol. 2010. 34:665–673.
36. Ramos E, Chen G, Shriner D, Doumatey A, Gerry NP, Herbert A, Huang H, Zhou J, Christman MF, Adeyemo A, et al. Replication of genome-wide association studies (GWAS) loci for fasting plasma glucose in African-Americans. Diabetologia. 2011. 54:783–788.
37. Chen WM, Erdos MR, Jackson AU, Saxena R, Sanna S, Silver KD, Timpson NJ, Hansen T, Orrù M, Grazia Piras M, et al. Variations in the G6PC2/ABCB11 genomic region are associated with fasting glucose levels. J Clin Invest. 2008. 118:2620–2628.
38. Shu XO, Long J, Cai Q, Qi L, Xiang YB, Cho YS, Tai ES, Li X, Lin X, Chow WH, et al. Identification of new genetic risk variants for type 2 diabetes. PLoS Genet. 2010. 6:pii: e1001127.
39. Ma RC, Tam CH, Wang Y, Luk AO, Hu C, Yang X, Lam V, Chan AW, Ho JS, Chow CC, et al. Genetic variants of the protein kinase C-beta 1 gene and development of end-stage renal disease in patients with type 2 diabetes. JAMA. 2010. 304:881–889.
40. Ikeda Y, Suehiro T, Osaki F, Tsuzura S, Kumon Y, Hashimoto K. Polymorphisms in the 5'-upstream region of the PKCbeta gene in Japanese patients with Type 2 diabetes. Diabet Med. 2004. 21:1113–1120.
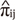 )] for the estimated proportion of alleles IBD
)] for the estimated proportion of alleles IBD 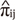 (i≠j) as dependent variables in the multivariate regression model. MERLIN-REGRESS software (8) was used for extended regression based QTL analysis. For this analysis, we controlled overestimated genetic variance in the model caused by MZ twins sharing identical genotypes within pairs.
(i≠j) as dependent variables in the multivariate regression model. MERLIN-REGRESS software (8) was used for extended regression based QTL analysis. For this analysis, we controlled overestimated genetic variance in the model caused by MZ twins sharing identical genotypes within pairs.



 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download