Abstract
Tinea incognito (TI) is a dermatophytic infection which has lost its typical clinical appearance because of improper use of steroids or calcineurin inhibitors. The incidence of TI is increasing nowadays. We conducted retrospective review on 283 patients with TI from 25 dermatology training hospitals in Korea from 2002-2010 to investigate the demographical, clinical, and mycological characteristics of TI, and to determine the associated risk factors. More than half (59.3%) patients were previously treated by non-dermatologists or self-treated. The mean duration of TI was 15.0 ± 25.3 months. The most common clinical manifestations were eczema-like lesion, psoriasis-like, and lupus erythematosus-like lesion. The trunk and face were frequently involved, and 91 patients (32.2%) also had coexisting fungal infections. Among 67 isolated strains, Trichophyton rubrum was the most frequently detected (73.1%). This is the largest study of TI reported to date and the first investigational report concerning TI in Korea. We suggest that doctors should consider TI when a patient has intractable eczema-like lesions accompanied by tinea pedis/unguium. Furthermore, there should be a policy change, which would make over-the-counter high-potency topical steroids less accessible in some countries, including Korea.
Tinea incognito (TI) is the term given to a dermatophyte infection, which has been modified in appearance by improper use of steroids or calcineurin inhibitors (1, 2). Since it was first described by Ive and Marks in 1968 (3), a few case reports and a number of review articles have been published on TI in English journals (1, 2, 4-6). Though typical dermatophytic infection on most skin surfaces except scalp, volar areas and nails usually present as annular lesions with erythematous scaly border and central clearing. Therefore, dermatophytic infection may be confused with other skin disorders such as granuloma annulare, discoid lupus erythematosus, pityriasis rosea, erythema annulare centrifugum, erythema migrans, or other dermatological lesions (7). Most textbooks or review articles state that mycological confirmation with laboratory testing before the start of antifungal therapy is recommended because the clinical diagnosis of fungal presence could be inaccurate (8). However, this is not easy to do in practice because of time pressure, inadequate access to equipment, lack of experience, low reproducibility, and so on (8, 9). Misdiagnosed dermatophytic disorders could be treated improperly with steroids. As some high-potency topical steroids are easily accessible as over-the-counter (OTC) products and non-dermatologists can also prescribe topical steroids freely without any fungal examination, the incidence of TI seems to be gradually increasing in Korea (10). However, there has been no published large-scale study on TI in Korea, as yet. For this reason, we investigated the demographics and past medical histories of TI patients, and clinical and mycological characteristics of TI in Korea.
In the period from 2002 to 2010, cases of TI were collected retrospectively from dermatologic departments of 25 general hospitals in Korea, which had a dermatology training program. We defined 4 criteria for diagnosis of TI: 1) loss of typical clinical appearance of tinea (Fig. 1A); 2) previous history of corticosteroid (topical or systemic) or calcineurin inhibitor application to present lesions; 3) positive for at least 1 mycological evaluation (KOH examination, mycologic culture, or skin biopsy with DPAS stain); and 4) improvement after antifungal treatment. To be enrolled in this study, all patients had to satisfy all the 4 diagnostic criteria listed above. Only the cases confined to dorsum of hands and feet were included to avoid confusion with tinea pedis and tinea manus.
The study protocol was approved by Institutional Review Boards or Ethics Committees of Pusan National University Hospital and informed consent was obtained.
Clinical data including charts and clinical photos from 25 hospitals were systematically and retrospectively reviewed. Demographic information included age, gender, coexisting diseases, and other dermatologic diseases. Past medical histories included the duration of TI, how the patients obtained the topical steroid or calcineurin inhibitor, and treatment modality. After dividing the patients into 3 groups (dermatologist-treated, non-dermatologist-treated, and self-treated TI groups), the duration of TI and treatment modality were compared among the 3 groups. Regarding the clinical characteristics of TI, the distribution, the most likely clinical feature, and coexisting fungal infections were investigated.
The KOH examination (20% potassium hydroxide) was performed to check for the presence of fungi. Mycological culture was performed on Sabouraud dextrose agar with chloramphenicol and cycloheximide. After incubation at 25℃ for at least 3 weeks, dermatophytes were identified by means of gross morphology, light microscopy, and/or biopsy with PAS stain (11).
Pearson's chi-square test was used to compare the frequency of treatment modalities and one-way ANOVA was used to compare the duration of TI among dermatologist-treated, non-dermatologist-treated, and self-treated TI patients group. A P value of less than 0.05 was considered statistically significant.
After thorough review, 283 patients fulfilled the diagnostic criteria of TI in this study. The mean age was 44.0 ± 22.5 yr (range 3-94) and 125 patients (44.3%) were female. Table 1 shows the age distribution of TI patients with a slightly lower frequency of patients with TI under 10 and over 80 yr old. Sixty-five patients (23.0%) had coexisting diseases at first clinic visit such as hypertension in 37 (13.1%), diabetes in 23 (8.1%), and hepatitis in 7 (2.5%). Five patients had underlying malignancy (1.8%), 2 patients suffered from angina, and 2 patients had asthma. In addition, 1 patient had adrenal insufficiency, 1 patient had myasthenia gravis, 1 had depression, and 1 had epilepsy. Sixteen patients (5.7%) had coexisting dermatologic diseases including 5 patients with atopic dermatitis (1.8%), 4 patients with psoriasis (1.4%), 3 with systemic lupus erythematosus (1.1%), and 2 with seborrheic dermatitis (0.7%). There was 1 patient with rosacea, and 1 patient with bullous pemphigoid.
The mean duration of TI in the study patients was 15.0±25.3 months. While mean duration of self-treated TI patients was 9.0±11.1 months, that of TI patients treated by dermatologists and non-dermatologists was 16.4±25.8 and 15.7±28.1 months, respectively. There was no statistical significance among the 3 groups (P = 0.234) (Table 2).
Before coming to the teaching hospital, 40.6% of TI patients received treatment from a dermatologist, 43.8% from non-dermatologists, and another 15.5% were self-treated. While all of self-treated patients used topical steroids only, people treated by dermatologists or non-dermatologists used various treatment modalities such as topical/systemic steroids, topical/systemic antibiotics, topical calcineurin inhibitor, steroid intralesional injection, or a combination of aforementioned agents. Overall, most of TI patients were treated with topical steroids only (86.9%), and other treatment modalities included topical and systemic steroids (6.4%), topical steroid and topical calcineurin inhibitor (1.4%), and topical calcineurin inhibitor (0.7%), etc. There were no significant differences in treatment modalities according to past physician's specialty (p > 0.05).
Overall, the trunk (30.4%) is the most commonly affected area of TI followed by the face (24.4%), foot (13.8%), multiple involvements (13.8%), the groin (9.9%), and hand (7.8%) (Table 3). The clinical features were variable, but regardless of distribution, over more than three-quarters of all study patients showed eczema-like (82.0%) lesions which included nonspecific eczema, contact dermatitis, seborrheic dermatitis, and atopic dermatitis. Less often, TI mimicked psoriasis (6.0%), lupus erythematosus (2.5%), impetigo (1.4%), urticaria (1.2%), folliculitis (0.7%), and other dermatological lesions (Table 3). According to the anatomical distribution, facial TI presented as eczema-like (76.8%), lupus erythematosus-like (8.7%), impetigo-like (2.9%), and vitiligo-like (2.9%) lesions. Trunk TI presented as eczema-like (79.1%) and psoriasis-like (10.5%) lesions, and almost all of groin, hand, and foot TI resembled eczema. When TI involved multiple sites, it appeared similar to eczema (69.2%), psoriasis (15.4%), folliculitis (2.6%), and other dermatological lesions (Table 3). In children, TI was most likely to be found in the facial area (11.6%), and the trunk (11.6%), and least likely to be found in the groin (3.6%).
In 91 cases (32.2%), other fungal diseases such as tinea pedis (42.9%), tinea unguium (31.9%), tinea pedis et unguium, or tinea unguium/tinea corporis (25.3%) were diagnosed apart from TI sites (Table 3). According to anatomical distribution, TI of the trunk, groin, or hand was commonly seen with tinea pedis (>50.0%), TI involving foot or multiple areas usually accompanied tinea unguium, and facial TI was strongly associated with tinea pedis et unguium.
Direct microscopic examination was performed in all cases and 260 cases (91.9%) were positive. Of 49 biopsied specimens, 42 (85.7%) showed fungal hyphae and/or spores by D-PAS stain. Sixty-seven cases (23.7%) were cultured in our study and Trichophyton rubrum was the most frequently detected dermatophyte (49/67, 73.%), regardless of TI distribution. Trichophyton mentagrophytes (6/67, 9.0%) and Microsporum canis (6/67, 9.0%) were the second-most frequently detected causative agents, and T. tonsurans, T. verrucosum and M. gypseum were also isolated in a few cases. While only 1 or 2 species of dermatophytes were found in groin, hand, and foot TI, various kinds of fungi were identified in face or trunk TI (Table 3).
Tinea incognito had been defined as tinea modified by the improper use of systemic or topical corticosteroids. However, as the use of topical calcineurin inhibitors has been increasing gradually in many dermatologic diseases such as atopic dermatitis, seborrheic dermatitis, intertriginous psoriasis, contact dermatitis and other dermatological lesions (12), the number of cases of modified tinea has also increased (7, 13, 14). Thus, we propose that TI be defined as certain dermatophytoses which have lost their usual clinical manifestation because of erroneous use of systemic/topical corticosteroids or topical calcineurin inhibitor, as in 1 recent article (2). In addition, we think that TI, which involves the hand or the foot, should be confined to the dorsal surface, because tinea pedis and tinea manus cannot be definitively differentiated from TI involving the palm or the sole. It has been suggested that the use of immunosuppressants decreases the fungus-induced local inflammation, and this may allow the fungus to grow slowly with less erythema or scaling causing a "modification" of the typical manifestation of tinea (7).
While TI seems to be common in dermatology practices currently, only a few numbers of large scale studies have been reported (1, 2, 4). These studies were done in Italy, Spain, and Iran. Our study in Korea was designed to be the largest scale study on TI. While previous case reports of TI in Korean literature (10, 15-21) (Table 4) showed female predominance, this study showed relatively equal gender distribution and relatively uniform age distribution (mean: 44.0±22.5 yr) were found except for patients over 80 yr. A recent article regarding TI in Italy (1) also reported equal gender distribution and similar mean age (42 yr), and another article in Iran (4) also revealed equal gender distribution with slightly younger mean age (32.6 yr). Based on these data, we can postulate that TI is common in middle-aged persons with little difference in gender. Moreover, 65 (23.0%) patients in our study had coexisting non-dermatologic diseases such as hypertension, diabetes, hepatitis, malignancy, and so on, and 16 (5.7%) patients had coexisting dermatologic disorders requiring systemic steroids or other immunosuppressants. This was lower than the previous Italian report in that 40% of patients with TI had non-dermatological pathologies which required treatment with systemic steroids (1). Though the percentage of the patients in this study who received immunosuppressive therapy was lower than in the Italian report, the possibility of TI should be kept in mind whenever the patient with skin lesions is on immunosuppressant medications.
There have been no published data regarding TI according to past treating physician's specialty or treatment modalities, as yet. Based on our study, over half of the patients were either treated by non-dermatologists (124/283, 43.8%) or self-treated (44/283, 15.5%). TI was thought to be associated with easy access to high-potency OTC topical steroids such as betamethasone valerate by patients and with lack of understanding of tinea by non-dermatologists. Therefore, there should be swift policy changes to limit OTC access of high-potency steroids to patients in Korea. This would limit inappropriate tinea treatment by patients. Furthermore, to reduce the number of cases of TI caused by non-dermatologists, education regarding skin diseases including fungal infections could be provided by Korean Dermatologic Associations.
Surprisingly, about 40% of the patients were treated by dermatologists in this study. Even though tinea can mimic many other skin disorders and there could be selection bias, this ratio seems to be too high. This may mean a lack of mycological evaluation and carelessness of dermatologists when diagnosing tinea infection. It is important for dermatologists to consider fungal infection in the differential diagnosis of skin disorders, and increase the use of laboratory tests for mycological evaluation. In practice, the medical cost for mycological examinations is very low in Korea. However, the patient load is high and doctors are pressed for time. This could be the prime reason for misdiagnosis of fungal infections (22). Therefore, we think that if physicians were better paid for mycological evaluations there might be more active mycological examinations and fewer misdiagnoses of fungal infections.
On the aspect of distribution, the trunk was the most commonly involved site of TI and the face was another commonly involved area, as reported in other original articles (1, 4) and previous Korean literatures. Another recent study regarding 54 childhood TI cases also reported similar results with highest incidence in the trunk and face (2), and our study backed it up with the same results. From these findings, we can postulate that the most common sites of TI are trunk and face regardless of age.
The clinical features of TI were reported to be variable, and the most prevalent features seen are eczema-like disorders such as nonspecific eczema, contact dermatitis, and atopic dermatitis (1, 4, 7), and previous Korean reports about TI also in accordance with it. Similarly, 232 TI patients in this study showed quite various clinical features such as eczema-like, psoriasis-like, lupus erythematosus-like, and etc. Specifically, nearly all cases of hand and foot TI showed eczema-like features. Therefore, when dealing with recalcitrant eczematous lesions on the hand or foot, mycological examination should always be considered. Compared to TI of groin, and hand and foot, where the eczema-like features were quite high, TI of the face, trunk, or multiple areas showed more variable features. Therefore, not only eczema-like lesions but also other recalcitrant skin manifestations resembling psoriasis, lupus erythematosus, impetigo, urticaria, etc., should also be carefully evaluated to rule out TI especially when skin diseases involve the face, the trunk, or multiple areas (Table 5).
Moreover, one-third of our study population (32.2%) had combined fungal diseases, which involved distant areas from present TI, and most of them had dermatophytic infection on their feet regardless of affected areas, including tinea pedis and tinea unguium. Therefore, in patients with refractory skin disease especially resembling eczema and also with concomitant tinea pedis or tinea unguium, TI should be ruled out because these coexisting fungal infections could be an autoinoculation source of superficial dermatophytic infection in another body part at any time.
As many previous studies confirmed (1, 23-25), Trichophyton rubrum (T. rubrum) was also the most frequently identified dermatophyte. T. rubrum is one of the anthropophilic dermatophytes and the most common pathogen in tinea corporis, tinea cruris, tinea manus, tinea pedis, and tinea unguium (26). Since TI affecting the trunk, groin, hands, and feet accounted for almost 60% of T1 in our study, it would not be surprising that T. rubrum was the most commonly isolated dermatophyte. Moreover, the high prevalence of combined tinea pedis and tinea unguium might also have contributed to the high isolation rate of T. rubrum. Anthropophilic dermatophytes have adapted to humans and elicit a mild to non-inflammatory host response unlike zoophilic and geophilic infections (26). This mild inflammatory response might be the cause of the long duration of TI because topical corticosteroids or topical calcineurin inhibitor could alleviate the inflammation, which could be the main mechanism of disguising the typical manifestation of tinea.
In summary, our research was the largest study of TI in Korea to date. We investigated the characteristics of TI according to the primary physician's specialty though the clinical and mycological results were similar to previous studies. From this study, we can suggest that long-lasting erythematous scaly skin lesions unresponsive to steroids or calcineurin inhibitor as the most important risk factors of TI. Not only truncal or facial involvement, but also combined tinea pedis/unguium or the history of immunosuppressant treatment could also be a good clue in diagnostic approach of TI. Moreover, we can suggest several things to reduce the incidence of TI based on our results. First, reform of OTC sales system of high-potency topical steroids is needed so that they are not as easily available to the public in some countries including Korea. Second, non-dermatologists need to be informed and educated that superficial dermatophytic infection could appear in a variety of forms. Care by experienced dermatologists could also be needed especially when dealing with long-lasting erythematous scaly skin lesions, which have proven to be unresponsive to steroids or calcineurin inhibitor treatment. Finally, we recommend dermatologists not to neglect TI as a possibility in cases of recalcitrant variable skin lesions, not hesitating to do active mycological examinations, which would give them some critical clues in diagnosis of TI, and doing careful clinical examinations when finding combined tinea pedis or tinea unguium.
Figures and Tables
Fig. 1
Various features of tinea incognito (A-G). Vitiligo-like (A; pre-treatment, B; after 4 weeks of application of topical pimecrolimus, C; 6 weeks after topical antifungal treatment), contact dermatitis-like (D), nonspecific eczema-like (E), seborrheic dermatitis-like (F), and lupus erythematosus-like (G) lesions.
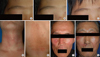
Table 2
Mean duration of the disease and previous treatment modalities according to past physician's specialty

References
1. Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006. 49:383–387.
2. del Boz J, Crespo V, Rivas-Ruiz F, de Troya M. Tinea incognito in children: 54 cases. Mycoses. 2011. 54:254–258.
3. Ive FA, Marks R. Tinea incognito. Br Med J. 1968. 3:149–152.
4. Ansar A, Farshchian M, Nazeri H, Ghiasian SA. Clinico-epidemiological and mycological aspects of tinea incognito in Iran: a 16-year study. Med Mycol J. 2011. 52:25–32.
5. Arenas R, Moreno-Coutino G, Vera L, Welsh O. Tinea incognito. Clin Dermatol. 2010. 28:137–139.
6. Lange M, Jasiel-Walikowska E, Nowicki R, Bykowska B. Tinea incognito due to Trichophyton mentagrophytes. Mycoses. 2009. 53:455–457.
7. Rallis E, Koumantaki-Mathioudaki E. Pimecrolimus induced tinea incognito masquerading as intertriginous psoriasis. Mycoses. 2008. 51:71–73.
8. Garcia-Doval I, Cabo F, Monteagudo B, Alvarez J, Ginarte M, Rodriguez-Alvarez MX, Abalde MT, Fernandez ML, Allegue F, Perez-Perez L, Florez A, Cabanillas M, Peon G, Zulaica A, Del Pozo J, Gomez-Centeno P. Clinical diagnosis of toenail onychomycosis is possible in some patients: cross-sectional diagnostic study and development of a diagnostic rule. Br J Dermatol. 2010. 163:743–751.
9. Rajpar SF, Abdullah A. Management of onychomycosis and awareness of guidelines among dermatologists. Br J Dermatol. 2006. 155:1080–1082.
10. Choi YL, Kim JA, Rho NK, Lee DY, Lee JH, Yang JM, Lee ES, Kim WS. A case of tinea incognito induced by 1% pimecrolimus (Elidel) cream. Korean J Dermatol. 2006. 44:731–733.
11. Hsiao YP, Lin HS, Wu TW, Shih HC, Wei SJ, Wang YL, Lin KL, Chiou HL, Yang JH. A comparative study of KOH test, PAS staining and fungal culture in diagnosis of onychomycosis in Taiwan. J Dermatol Sci. 2007. 45:138–140.
12. Wollina U, Hansel G, Koch A, Abdel-Naser MB. Topical pimecrolimus for skin disease other than atopic dermatitis. Expert Opin Pharmacother. 2006. 7:1967–1975.
13. Crawford KM, Bostrom P, Russ B, Boyd J. Pimecrolimus-induced tinea incognito. Skinmed. 2004. 3:352–353.
14. Siddaiah N, Erickson Q, Miller G, Elston DM. Tacrolimus-induced tinea incognito. Cutis. 2004. 73:237–238.
15. Yang CW, Lee BG, Lee MH, Kim NI. A case of tinea incognito. Korean J Dermatol. 1989. 27:79–82.
16. Kang HY, Son HC, Lim YS, Cho YW, Han JY. A case of tinea incognito on the face due to Trichophyton mentagrophytes. Korean J Dermatol. 2000. 38:1124–1126.
17. Kim KJ, Jee MS, Choi JH, Sung KJ, Moon KC, Koh JK. A case of tinea incognito presented as folliculitis. Korean J Dermatol. 2001. 39:1328–1330.
18. Han TY, Rho YK, Seo SJ, Hong CK, Song KY. A case of tinea incognito presented like furunculosis. Korean J Med Mycol. 2008. 13:138–141.
19. Park SB, Lee YW, Park EJ, Kwon IH, Kim KH, Kim KJ. A case of tinea faciei caused by Trichophyton mentagrophytes with atypical presentation. Korean J Med Mycol. 2010. 15:170–174.
20. Hwang SM, Kim DM, Suh MK, Ha GY, Kim JR. Eczema-like tinea incognito occurring leg. Korean J Med Mycol. 2011. 16:51–55.
21. Lee JS, Cho YS, Song KH, Hwang SR, Park J, Yun SK, Kim HU. Tinea incognito with changes in clinical feature related to antifungal treatment. Korean J Med Mycol. 2011. 16:118–123.
22. Kim WJ, Song M, Kim HS, Kim SH, Ko HC, Kim BS, Kim MB. Various nail disorders misdiagnosed and treated as onychomycosis. Korean J Dermatol. 2011. 49:408–414.
23. Szepietowski JC, Matusiak L. Trichophyton rubrum autoinoculation from infected nails is not such a rare phenomenon. Mycoses. 2008. 51:345–346.
24. Nenoff P, Mugge C, Herrmann J, Keller U. Tinea faciei incognito due to Trichophyton rubrum as a result of autoinoculation from onychomycosis. Mycoses. 2007. 50:Suppl 2. 20–25.
25. Serarslan G. Pustular psoriasis-like tinea incognito due to Trichophyton rubrum. Mycoses. 2007. 50:523–524.
26. Schieke SM, Garg A. Fungal disease. Fitzpatrick's dermatology in general medicine. 2012. 8th ed. NewYork: McGraw-Hill;2277–2297.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download