Abstract
Background
High asymmetrical dimethylarginine (ADMA) levels have been associated with endothelial dysfunction and contribute to the development of several diseases. However, data on the relationship between hepatitis B virus (HBV) and ADMA are limited. The aim of our study was to explore the relationship between ADMA and HBV by comparing the ADMA levels in patients with chronic active hepatitis B (CHB), inactive HBV carriers (carriers), and healthy volunteers (controls).
Methods
The participants were divided into three groups: 90 patients with CHB, 90 HBV carriers, and 90 controls. Serum ADMA levels were quantified using an ELISA kit (Cusabio, Wuhan, China). The data were analyzed using an ANOVA or the Kruskal-Wallis test as appropriate, with P<0.05 considered significant.
Nitric oxide synthase (NOS) plays a fundamental role in maintaining the vascular structure [12], and its main function is the synthesis of NO from L-arginine, an amino acid in the vascular endothelium. As the main inhibitor of NOS, asymmetrical dimethylarginine (ADMA) is an endogenous molecule detected in human blood and urine. Almost 90% of the ADMA present is metabolized by dimethylarginine dimethylaminohydrolase in the vascular endothelium of the liver and kidney, where ADMA prevents the cellular ingestion of L-arginine by inhibiting the activity of NOS. Accordingly, high ADMA levels have been associated with endothelium dysfunction and play a role in the development of various conditions [345].
Changes in serum ADMA levels have been associated with many conditions, including cardiovascular system diseases, diabetes mellitus, multiple organ failure, chronic renal failure, hyperthyroidism, preeclampsia and neonatal sepsis [567891011], as well as in certain infectious diseases such as brucellosis [12]. Similarly, ADMA levels have been observed to change after interferon-alpha (IFN-α) treatment in patients with chronic hepatitis C virus (HCV) infection [13]. However, the association of ADMA with hepatitis B virus (HBV) infection remains unclear.
HBV is an important health problem worldwide. At present, one third of the world's population has serologic evidence of past or present HBV infection, and 400 million people have been reported to be chronically infected with this agent [141516]. Although HBV is a hepatotropic virus, it also causes extrahepatic symptoms via various mechanisms, including vasculitis [17]. However, to our knowledge, no study has examined the ADMA levels in patients infected with HBV. Thus, the nature of the relationship between HBV causing vasculitis and ADMA, which is known to be involved in endothelium dysfunction, remains to be elucidated. Accordingly, we aimed to determine the ADMA levels in patients with chronic active hepatitis B (CHB), inactive HBV carriers (carriers), and healthy volunteers (controls), and to explore the relationship between ADMA and HBV.
This prospective study was carried out in the Department of Infectious Diseases and Clinical Microbiology of the Faculty of Medicine, Erzincan University, Turkey, between January 2013 and December 2015. A total of 270 participants were included in the study and divided into three groups: 90 patients with CHB (mean age; 40.97±13.89 years), 90 carriers (41.73±14.85 years), and 90 controls (34.24±8.42 years). Patients with CHB were selected among patients who were antiviral-naive with an HBV DNA level of >2,000 IU/mL. Liver biopsy was performed for these patients. Fibrosis and the histological activity index (HAI) were scored using the Ishak scoring system [18]. Stages of fibrosis ranged from 0 to 6. In this system, the fibrosis scores are defined as follows: 0, no fibrosis; 1, fibrous expansion of some portal areas, with or without short fibrous septa; 2, fibrous expansion of most portal areas, with or without short fibrous septa; 3, fibrous expansion of most portal areas with occasional portal to portal bridging; 4, fibrous expansion of most portal areas with marked bridging; 5, marked bridging with occasional nodules (incomplete cirrhosis); and 6, cirrhosis, probable or definite cirrhosis [18]. Based on the Ishak score, patients with a HAI of at least 6 and/or a fibrosis score of at least 2 were included in the study. Patients who were HBV surface antigen (HBsAg)-positive for more than six months, with normal liver function test results, and an HBV DNA level of <2,000 IU/mL were included in the carrier group. Lastly, 90 healthy volunteers with no complaints were enrolled as the control group. Patients with any comorbidities that could potentially affect ADMA levels such as cardiovascular system diseases, diabetes mellitus, multiple organ failure, or chronic renal failure were excluded. The demographic characteristics, including sex, age, and residential address, of all groups were recorded. All were subjected to physical examinations to assess the presence of jaundice, signs of chronic liver disease, hepatosplenomegaly, ascites and AST, ALT, bilirubin, platelet, albumin, and alpha feto protein (AFP) levels.
Ethical approval for this study was obtained from the Department of Ethics Committee, Rectorate of Erzincan University, with the decision dated February 10, 2015 (approval number 01/05). All participants provided written informed consent.
Venous blood samples were obtained from all participants. Serum was separated from the blood after resting in a test tube for about 2 hours at 25℃ followed by centrifugation at 1,000×g for 15 minutes, and then stored at −20℃ until used for the ELISA.
Serum ADMA levels were quantified with an ELISA kit (Cusabio, Wuhan, China). The detection range of the kit was 7.8–500 ng/mL. All assay procedures were carried out according to the manufacturer's instructions. The absorbance values of standards and samples were obtained at 450 nm (reference wavelength 540–570 nm) using an Epoch spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
First, descriptive statistics for the continuous parameters were obtained, expressed as mean±standard deviation. Data were analyzed using Shapiro-Wilk and Levene tests. For comparisons among groups, a one-way ANOVA and the Tukey honestly significant difference (HSD) multiple comparison test were used. When the tests for parametric assumptions fell short, the non-parametric Kruskal-Wallis test was used followed by multiple comparison correction with the Bonferroni-Dunn test. SPPS 20 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY, USA) was used for all analyses. P<0.05 was considered statistically significant.
The laboratory data, including AST, ALT, and AFP levels, of all participants are summarized in Table 1. The mean AST and ALT levels in patients with CHB were significantly higher (P<0.001) than those of carriers and controls, with no differences between the carriers and controls. In contrast, the mean platelet level was significantly lower in patients with CHB than in carriers and controls (P<0.001) (Table 1).
Demographic characteristics of all participants, including sex ratio, age, and serum ADMA levels are summarized in Table 2. ADMA levels were significantly higher in patients with CHB than in carriers and controls (P<0.01, respectively), but they did not significantly differ between carriers and controls. In addition, the clinical score and ADMA levels of the patients were correlated (Table 2).
Plasma ADMA levels have been reported to be particularly elevated in patients with liver cirrhosis, alcoholic hepatitis, and acute liver failure [192021].
Moreover, high plasma ADMA levels have been detected in patients with alcoholic hepatitis and high portal venous pressure, which were further associated with liver injury [22]. In addition, ADMA levels increased after IFN-α treatment in patients with chronic HCV infection [1113], and high ADMA levels were also detected in patients with HCV-HIV co-infection; however, biological markers related to endothelium function did not differ after antiretroviral treatment in these patients [232425].
To our knowledge, this is the first study to compare ADMA levels among patients with CHB, carriers, and controls. We found that ADMA levels were significantly higher in patients with CHB than in carriers and controls. In addition, ADMA levels did not significantly differ between carriers and controls.
In another study, the plasma levels of NO and ADMA were not affected in patients with chronic HCV without acute inflammatory activity signs [26]. Similarly, we detected higher ADMA levels in patients with CHB with inflammation and liver injury. In parallel, in the carrier group with little to no liver injury or inflammation, the ADMA levels were similar to those of controls.
Nevertheless, there are many open questions related to the pathological changes in the liver occurring in patients with CHB. However, a relationship between HBV, which causes vascular pathologies such as vasculitis in addition to pathologies in the liver, and ADMA, which is known to be involved in the pathogenesis of vasculitis, is expected [17]. Thus, research on elevation in ADMA levels will provide opportunities to establish new interpretations of the role of HBV in vasculitis, with new contributions to the development of novel treatment methods.
In conclusion, our study provides evidence that serum ADMA levels were significantly higher in patients with CHB than in carriers and controls. Thus, further research should be conducted on the relationship between ADMA and HBV infection.
Acknowledgments
This project was financially supported by the Scientific Research and Project Unit of Erzincan University (Project No: SAG-A-240215-0125).
References
1. Aldámiz-Echevarría L, Andrade F. Asymmetric dimethylarginine, endothelial dysfunction and renal disease. Int J Mol Sci. 2012; 13:11288–11311. PMID: 23109853.
2. Liu X, Hou L, Xu D, Chen A, Yang L, Zhuang Y, et al. Effect of asymmetric dimethlarginine (ADMA) on heart failure development. Nitric Oxide. 2016; 54:73–81. PMID: 26923818.
3. Tain YL, Hsu CN. Toxic dimethylarginines: asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA). Toxins (Basel). 2017; 9:E92. DOI: 10.3390/toxins9030092. PMID: 28272322.
4. McCarty MF. Asymmetric dimethylarginine is a well-established mediating risk factor for cardiovascular morbidity and mortality-should patients with elevated levels be supplemented with citrulline? Healthcare (Basel). 2016; 4:E40. DOI: 10.3390/healthcare4030040. PMID: 27417628.
5. Erbil MK, Kurt YG, Yaman H, Çaır E, Akgül EÖ, Çaycı T. Metabolism of asymmetric dimethylarginine and its clinical significance. Turk J Bioch. 2012; 37:99–105.
6. El Dayem SM, Battah AA, El-Shehaby A, El Bohy AE. Asymmetric dimethyl L-arginine, nitric oxide and cardiovacular disease in adolescent type 1 diabetics. J Pediatr Endocrinol Metab. 2014; 27:437–444. PMID: 24468606.
7. Taşkıran B, Uğur Altun B, Vardar SA, Demir AM, Karadağ ÇH, Altun A. Effect of exercise on ADMA level in type 2 diabetes mellitus. Balkan Med J. 2012; 29:62–67.
8. Sitar ME. Asymmetric dimethylarginine and its relation as a biomarker in nephrologic diseases. Biomark Insights. 2016; 11:131–137. PMID: 27980388.
9. Ittermann T, Bahls M, Atzler D, Friedrich N, Schwedhelm E, Böger RH, et al. L-Arginine derivatives are associated with the hyperthyroid state in the general population. Thyroid. 2016; 26:212–218. PMID: 26650143.
10. Aydemir O, Ozcan B, Yucel H, Bas AY, Demirel N. Asymmetric dimethylarginine and L-arginine levels in neonatal sepsis and septic shock. J Matern Fetal Neonatal Med. 2015; 28:977–982. PMID: 24983667.
11. Ferrigno A, Di Pasqua LG, Berardo C, Richelmi P, Vairetti M. Liver plays a central role in asymmetric dimethylarginine-mediated organ injury. World J Gastroenterol. 2015; 21:5131–5137. PMID: 25954086.
12. Mengeloglu Z, Sünnetcioglu M, Tosun M, Kücükbayrak A, Ceylan MR, Baran AI, et al. High asymmetric dimethylarginine (ADMA) levels in patients with brucellosis. Inflammation. 2014; 37:127–131. PMID: 23978912.
13. Baranyi A, Meinitzer A, Putz-Bankuti C, Stauber R, Kapfhammer HP, Rothenhäusler HB. Asymmetric dimethylarginine responses during interferon-α-induced depression in patients with chronic hepatitis C infection. Psychosom Med. 2014; 76:197–207. PMID: 24608038.
14. Hu J, Liu K. Complete and incomplete hepatitis B virus particles: formation, function, and application. Viruses. 2017; 9:E56. PMID: 28335554.
15. Sandhu P, Haque M, Humphries-Bickley T, Ravi S, Song J. Hepatitis B virus immunopathology, model systems, and current therapies. Front Immunol. 2017; 8:436. PMID: 28450868.
16. An J, Lim YS, Kim GA, Han SB, Jeong W, Lee D, et al. Telbivudine versus entecavir in patients with undetectable hepatitis B virus DNA: a randomized trial. BMC Gastroenterol. 2017; 17:15. PMID: 28103819.
17. Joshi U, Subedi R, Gajurel BP. Hepatitis B virus induced cytoplasmic antineutrophil cytoplasmic antibody-mediated vasculitis causing subarachnoid hemorrhage acute transverse myelitis, and nephropathy: a case report. J Med Case Rep. 2017; 11:91. PMID: 28366165.
18. Ishak K, Baptista A, Bianch L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995; 22:696–699. PMID: 7560864.
19. Lluch P, Segarra G, Medina P. Asymmetric dimethylarginine as a mediator of vascular dysfunction in cirrhosis. World J Gastroenterol. 2015; 21:9466–9475. PMID: 26327755.
20. Nicković V, Nikolić J, Djindjić N, Ilić M, Nicković J, Mladenović D, et al. Diagnostical significance of dimethylarginine in the development of hepatorenal syndrome in patients with alcoholic liver cirrhosis. Vojnosanit Pregl. 2012; 69:686–691. PMID: 22924265.
21. Brenner T, Flemin TH, Rosenhagen C, Krauser U, Mieth M, Bruckner T, et al. L-arginine and asymmetric dimethylarginine are early predictors for survival in septic patients with acute liver failure. Mediators Inflamm. 2012; 2012:210454. PMID: 22619480.
22. Mookerjee RP, Malaki M, Davies NA, Hodges SJ, Dalton RN, Turner C, et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in several alcoholic hepatitis. Hepatology. 2007; 45:62–71. PMID: 17187433.
23. Beltrán LM, Hernández RM, de Pablo Bernal RS, Morillo JSG, Egido J, Noval ML, et al. Reduced sTWEAK and increased sCD163 levels in HIV-infected patients: modulation by antiretroviral treatment, HIV replication and HCV co-infection. PLoS One. 2014; 9:e90541. PMID: 24594990.
24. Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008; 5:e203. PMID: 18942885.
25. Kristoffersen US, Kofoed K, Kronborg G, Giger AK, Kjaer A, Lebech AM. Reduction in circulating markers of endothelial dsyfunction in HIV-infected patients during antiretroviral therapy. HIV Med. 2009; 10:79–87. PMID: 19200170.
26. Lluch P, Cortina B, Vila JM, Segarra G, Mauricio MD, Del Olmo JA, et al. Unchanged plasma levels of dimethylarginines and nitric oxide in chronic hepatitis C. Scand J Gastroenterol. 2009; 44:224–228. PMID: 18951278.
Table 1
Laboratory findings of the study groups
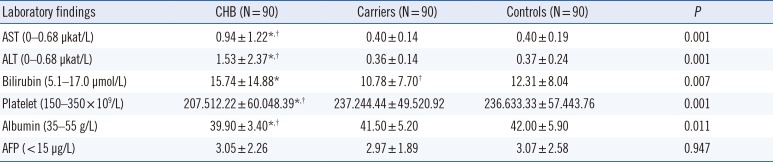
Table 2
Demographic characteristics and ADMA levels of the study groups
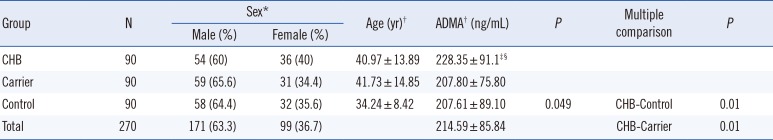
All data were analyzed using one-way ANOVA followed by Tukey's honestly significant difference (HSD) test for multiple comparisons.
*Values are presented as N (percentage); †Values are presented as mean±standard deviation; ‡Significant difference from controls; §Significant difference from carriers.
Abbreviations: CHB, chronic active hepatitis B; ADMA, asymmetric dimethylarginine.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download