Abstract
Herein, we reported the phytochemical investigation of whole thallus Sumatran lichen, Stereocaulon graminosum Schaer, and isolated a mono aromatic compound, ethyl haematommate (1). The structure of compound 1 have been established based on spectroscopic data and confirmed by single crystal X-ray structure analysis.
References
(1). Singh R.., Ranjan S.., Nayaka S.., Pathre U. V.., Shirke P. A.Acta Physiol. Plant. 2013. 35:1605–1615.
(2). Ismed F.., Lohézic-Le Dévéhat F.., Delalande O.., Sinbandhit S.., Bakhtiar A.., Boustie J.Fitoterapia. 2012. 83:1693–1698.
(3). Ismed F.., Lohézic-Le Dévéhat F.., Rouaud I.., Ferron S.., Bakhtiar A.., Boustie J. Z.Naturforsch. C. 2017. 72:55–62.
(4). Stocker-Wörgötter E.Nat. Prod. Rep. 2008. 25:188–200.
(5). Seshadri T. R.Proc. Indian Acad. Sci. 1944. 20:1–14.
(6). Marante F. J. T.., Castellano A. G.., Rosas F. E.., Aguiar J. Q.., Barrera J. B. J.Chem. Ecol,. 2003. 29:2049–2071.

(7). Choudhary M. I.., Ali M.., Wahab A.., Khan A.., Rasheed S.., Shyaula S. L.., Rahman A.Sci. China Chem. 2011. 54:1926–1931.
(8). Manojlovic N. T.., Vasiljevic P. J.., Maskovic P. Z.., Juskovic M.., Bogdanovic-Dusanovic G.Evid. Based Complement. Alternat. Med. 2012. 2012:452431.
(9). Huneck S.., Yoshimura I.Identification of lichen substances. Springer;United States: 1996. . pp.p. 421–422.
(10). Calculated using ABSCOR. Empirical Absorption Correction Based on Fourier Series Approximation; Rigaku: Texas, 1994. Based on Fourier Series Approximation; Rigaku: Texas,. 1994.
(11). Burla M. C.., Caliandro R.., Camalli M.., Carrozzini B.., Cascarano G. L.., De Caro L.., Giacovazzo C.., Polidori G.., Spagna R. J.Appl. Cryst. 2005. 38:381–388.
(12). Sheldrick G. M.Acta Crystallogr. A. 2008. 64:112–122.
(13). Macrae C. F.., Bruno I. J.., Chisholm J. A.., Edgington P. R.., McCabe P.., Pidcock E.., Rodriguez-Monge L.., Taylor R.., van de Streek J.., Wood P. A. J.Appl. Cryst. 2008. 41:466–470.
(14). Rao P. S.., Sarma K. G.., Seshadri T. R.Proc. Indian Acad. Sci. 1967. 66:1–14.
(15). Putra O. D.., Furuishi T.., Yonemochi E.., Terada K.., Uekusa H.Cryst. Growth Des. 2016. 16:3577–3581.
(16). Ismed F.., Farhan A.., Bakhtiar A.., Zaini E.., Nugraha Y. P.., Dwichandra Putra, O. Uekusa H.Acta Crystallogr. E Crystallogr. Commun. 2016. 72:1587–1589.
(17). Putra O. D.., Umeda D.., Nugraha Y. P.., Furuishi T.., Nagase H.., Fukuzawa K.., Uekusa H.., Yonemochi E.CrystEngComm. 2017. 19:2614–2622.
(18). Groom C. R.., Bruno I. J.., Lightfoot M. P.., Ward S.C.; Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016. 72:171–179.
Fig. 3.
Thermal ellipsoid structures of compound 1 with atom labelling drawn at a 50% probability level. The asymmetric unit contained one molecule of compound 1.
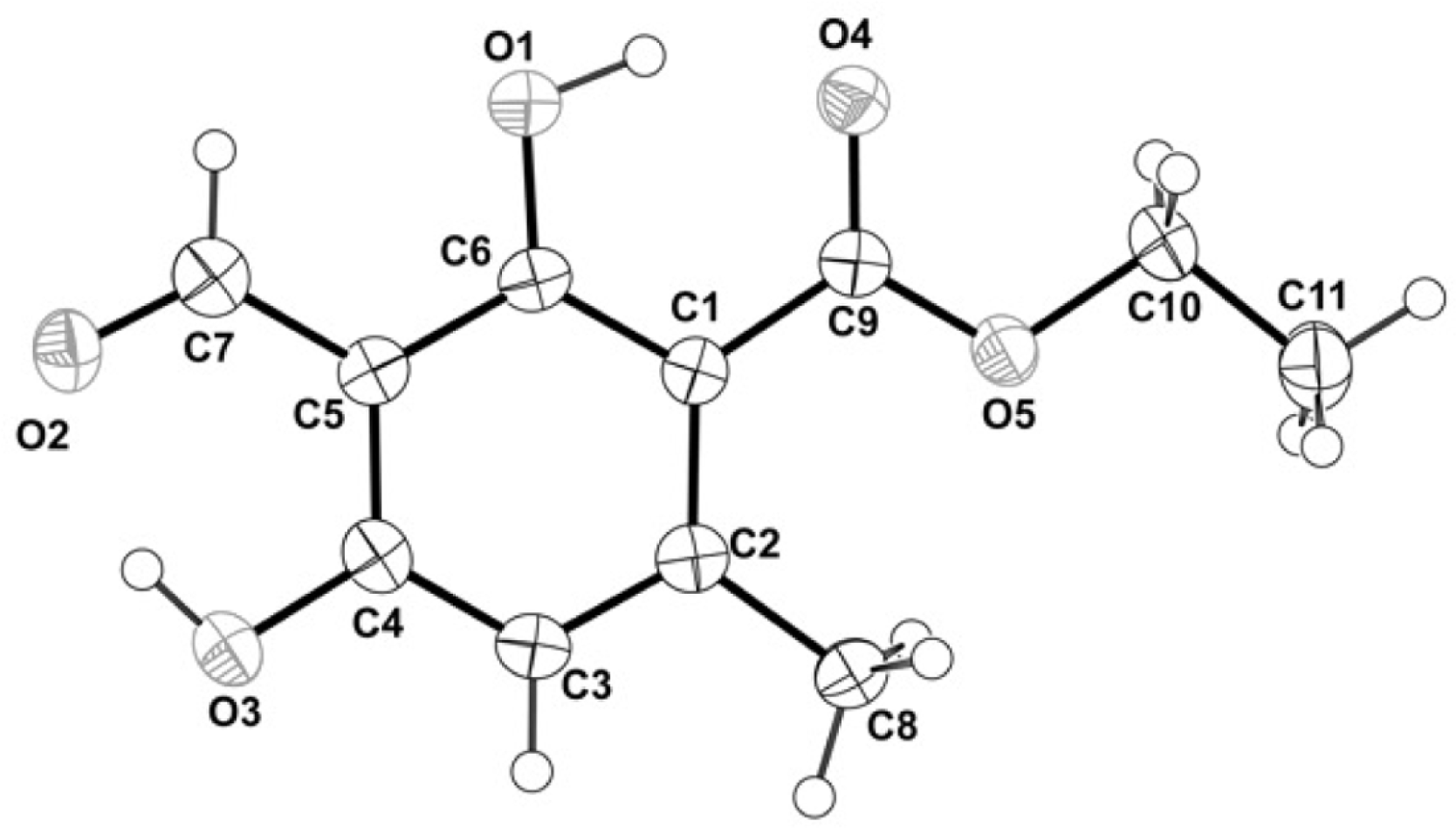
Fig. 4.
The π–π interactions in compound 1 create a layered architecture propagating in the (001) plane. Hydrogen atoms are omitted for clarity.
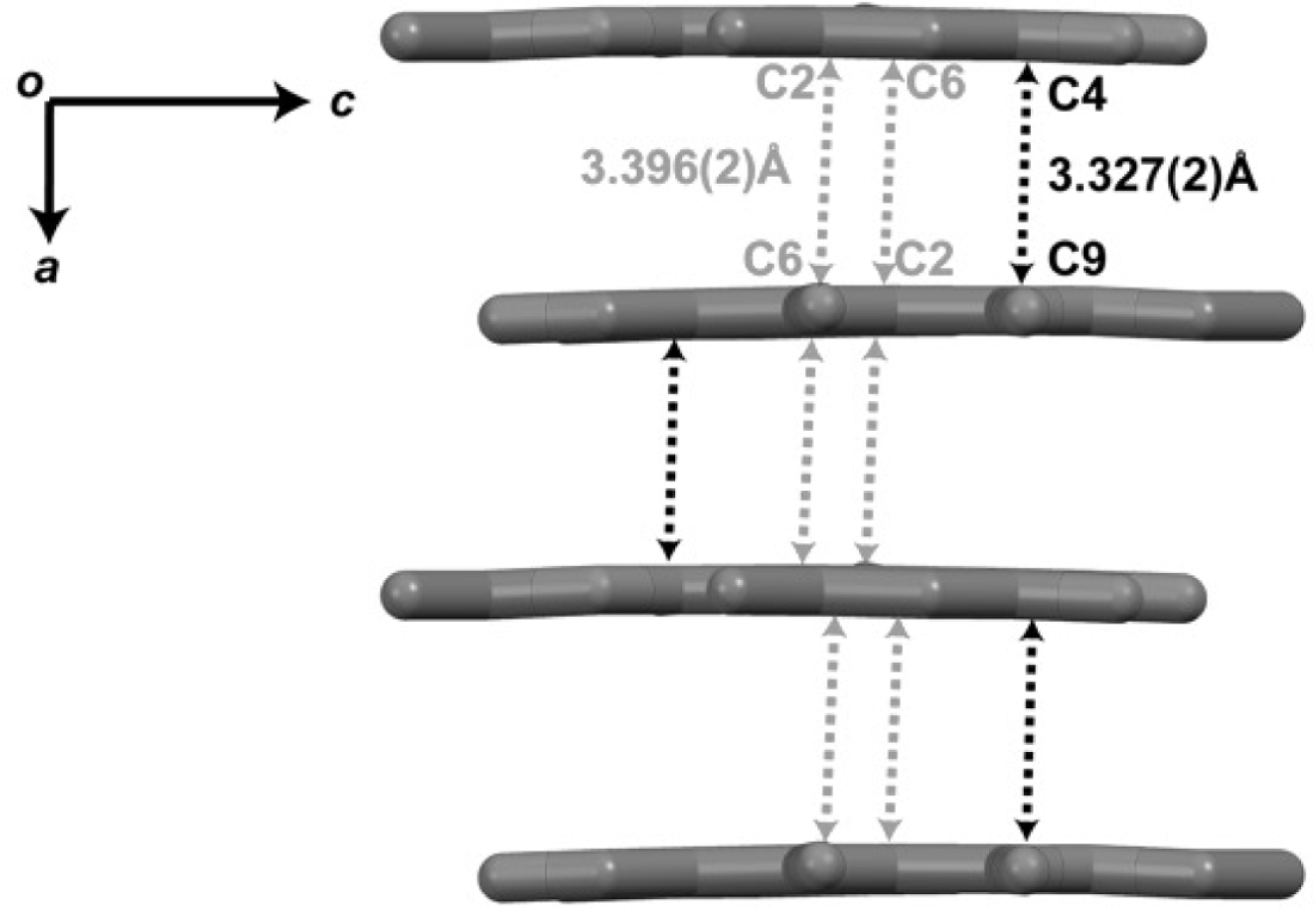
Table 1.
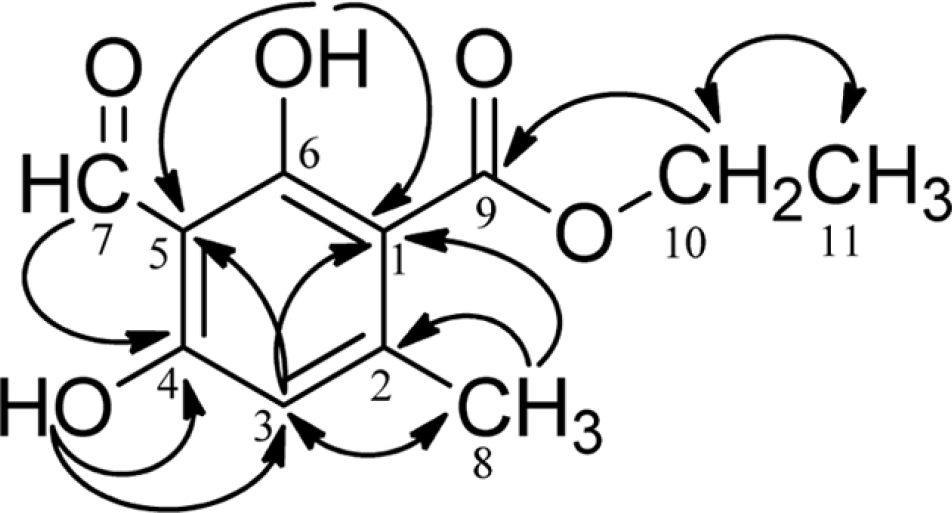
1H- and 13C-NMR data of ethyl haematommate (1) in CDCl3 (δ in ppm, 500 MHz for 1H and 125 MHz for 13C)a
Table 2.
Crystallographic data of compound 1




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download