Abstract
Clitoria ternatea or Commonly known blue pea, is a perennial climber crop native to Asian countries. The current study was aimed to evaluate the antimicrobial activity C. ternatea extract on food borne microorganisms and its antifungal effect on Penicillium expansum. The extract showed significant antimicrobial activity against 3 Gram positive bacteria, 2 Gram negative bacteria and 1 filamentous fungus on disc diffusion assay. The extract also showed good biocidal effect on all Gram positive bacteria tested and P. expansum. However, the kill curve analysis revealed that the fungicidal activity of the extract against P. expansum conidia was depend on the concentration of the extract and the time of exposure of the conidia to the extract. The scanning electron micrograph of the extract treated P. expansum culture showed alterations in the morphology of fungal hyphae. The germination of P. expansum conidia was completely inhibited and conidial development was totally suppressed by the extract, suggesting the possible mode of action of anthocyanin. Besides, the extract also exhibited 5.0-log suppression of microbial growth relative to control in the rice model. The results indicate the potential use of the C. ternatea anthocyanin as food biopreservative.
References
(1). Lima A. A. N. D.., Sobringho J. L. S.., Lyra M. A. M. D.., Santos F. L. A. D.., Figueiredo C. B. M.., Neto P. J. R.Int. J. Pharm. Pharm. Sci. 2015. 7:371–375.
(2). Kagan C. R.ACS Nano. 2016. 10:2985–2986.
(3). Kawai T.., Sekizuka T.., Yahata Y.., Kuroda M.., Kumeda Y.., Iijima Y.., Kamata Y.., Sugita-Konishi Y.., Ohnishi T.Clin. Infect. Dis. 2012. 54:1046–1052.
(4). Abdul-Mutalib N. A.., Nordin S. A.., Osman M.., Ishida N.., Tashiro K.., Sakai K.., Tashiro Y.., Maeda T.., Shirai Y.Int. J. Food Microbiol. 2015. 200:57–65.
(5). Spadaro D.., Lorè A.., Garibaldi A.., Gullino M. L.Postharvest Biol. Technol. 2013. 75:1–8.
(6). Yu C.., Zhou T.., Sheng K.., Zeng L.., Ye C.., Yu T.., Zheng X.Int. J. Food Microbiol. 2013. 164:155–160.
(7). Bhatia M.., Chahal J.., Gupta S.Int. J. Pharm. Sci. Res. 2014. 5:600–606.
(8). Kungsuwan K.., Singh K.., Phetkao S.., Utama-ang N.Food Appl. Biosci. J. 2014. 2:31–46.
(9). Chong F. C.., Gwee X. F.Nat. Prod. Res. 2015. 29:1485–1487.
(10). Priya F. J.., Vimala J. R.., Bama R. S.., Lavanya M.World J. Pharm. Pharm. Sci. 2016. 5:753–765.
(11). Tong W. Y.., Ang S. N.., Darah I.., Latiffah Z.World J. Pharm. Pharm. Sci. 2014. 3:121–132.
(12). Darah I.., Chong C. L.., Tong W. Y.., Latiffah Z.., Lim S.Trop. J. Pharm. Res. 2015. 14:2091–2097.
(13). Dalla Lana D. F.., Donato R. K.., Bündchen C.., Guez C. M.., Bergamo V. Z.., de Oliveira L. F.., Machado M. M.., Schrekker H. S.., Fuentefria A. M. J.Appl. Microbiol. 2015. 119:377–388.
(14). Biswas B.., Rogers K.., McLaughlin F.., Daniels D.., Yadav A.Int. J. Microbiol. 2013. 2013:1–7.
(15). Kamilla L.., Mnsor S. M.., Ramanathan S.., Sasidharan S.Pharm. Online. 2009. 1:731–738.
(16). Nair V.., Bang W. Y.., Schreckinger E.., Andarwulan N.., Cisneros-Zevallos L. J.Agric. Food Chem. 2015. 63:6355–6365.
(17). Kondo T.., Ueda M.., Goto T.Tetrahedron. 1990. 46:4749–4756.
(18). Rao V. S.., Santos F. A.., Sobreira T. T.., Souza M. F.., Melo C. L.., Silveira E. R.Planta Med. 1997. 63:146–149.
(19). Nikaido H.Microbiol. Mol. Biol. Rev. 2003. 67:593–656.
(20). Palazzo I. C. V.., Araujo M. L. C.., Darini A. L. C. J.Clin. Microbiol. 2005. 43:179–185.
(21). Kamilla L.., Mansor S. M.., Ramanathan S.., Sasidharan S.Microsc. Microanal. 2009. 15:366–372.
(22). Moussa S. H.., Tayel A. A.., Al-Hassan A. A.., Farouk A. J.Mycol. 2013. 2013:753692.
(23). Rafiee E.., Khodayari M.., Shahebrahimi S.., Joshaghani M. J.Mol. Catal. A. 2011. 351:204–209.
Fig. 1.
The kill curve of C. ternatea extract on P. expansum. The fungicidal activity of the extract was concentration-dependent.
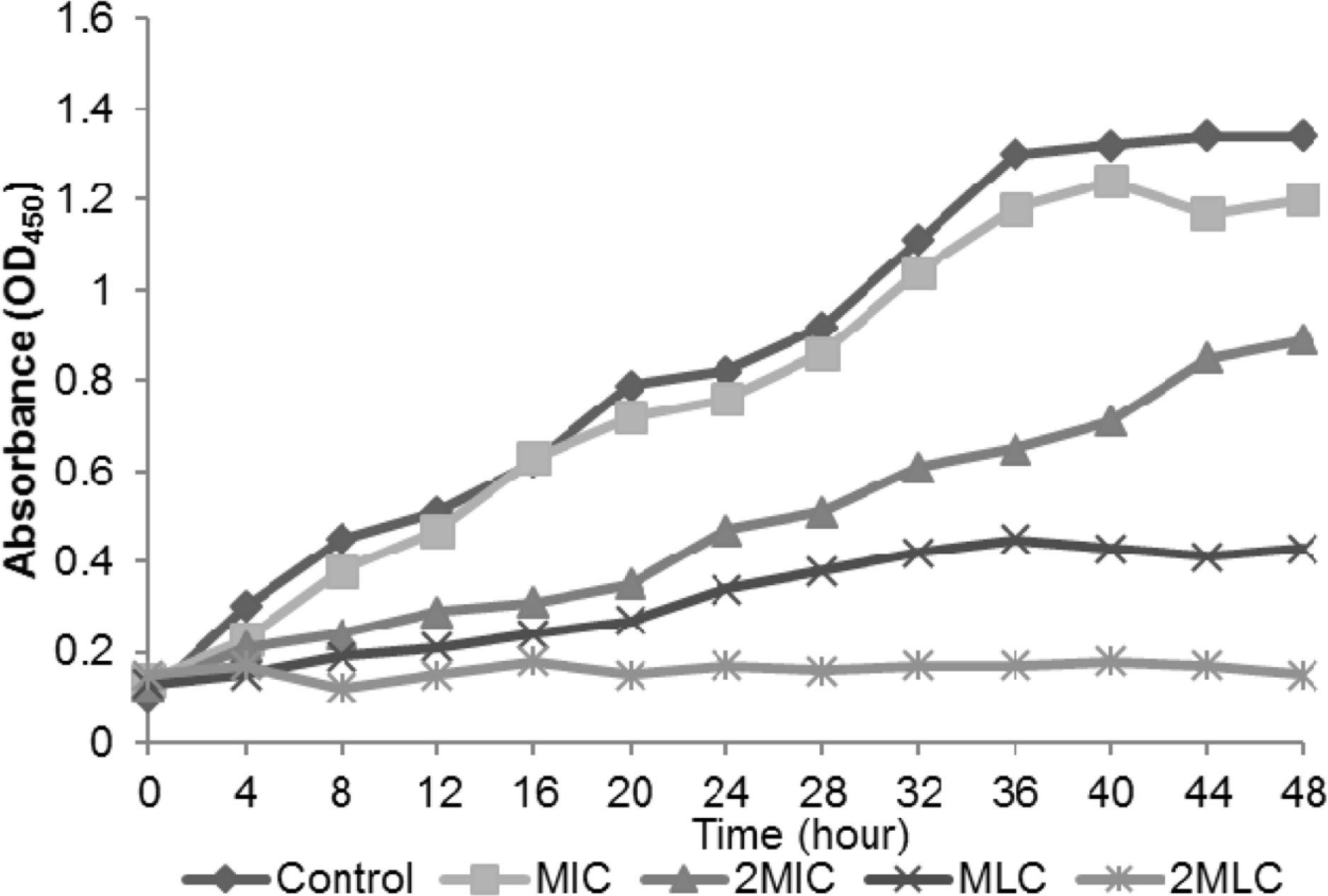
Fig. 2.
SEM micrograph of food borne P. expansum treated with (A) sterile distilled water (B) C. ternatea extract (5000× magnification). The results suggest the irreversible damage on the conidiophore of P. expansum.
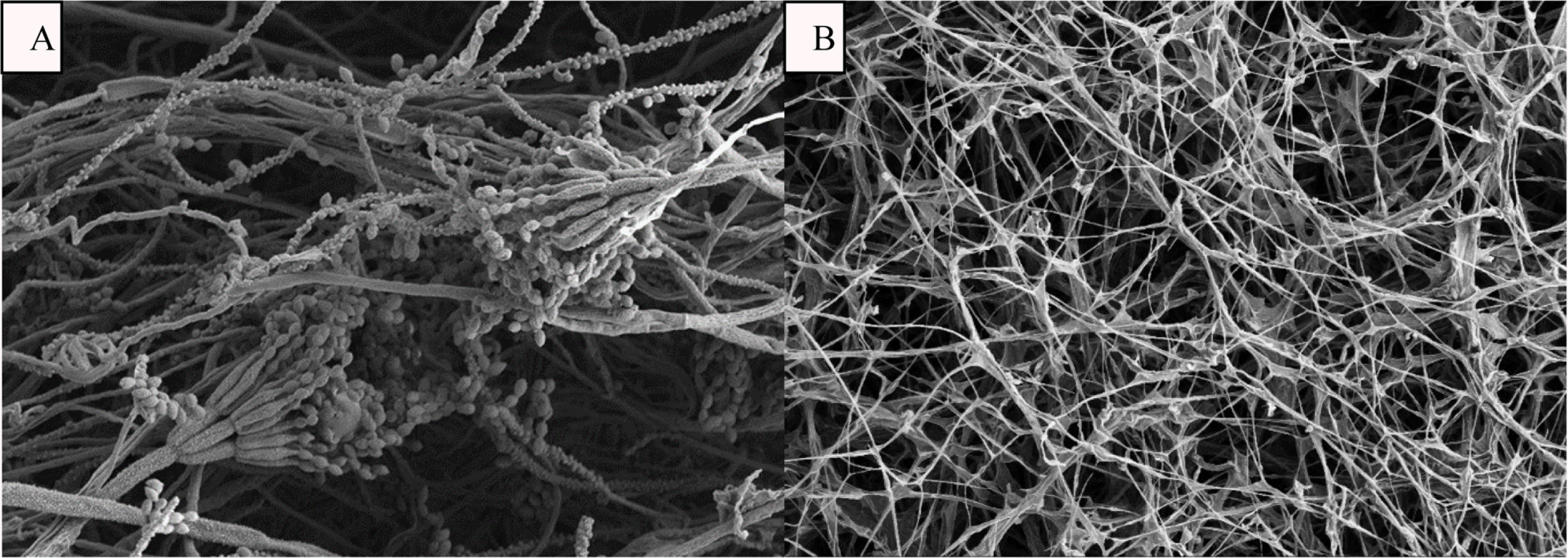
Fig. 3.
The microbial load of the food model obtained throughout the 5 days of incubation period. Test substance: (◆) C. ternatea extract, (■) Distilled water control. The extract significantly inhibits the microbial growth in the rice model.
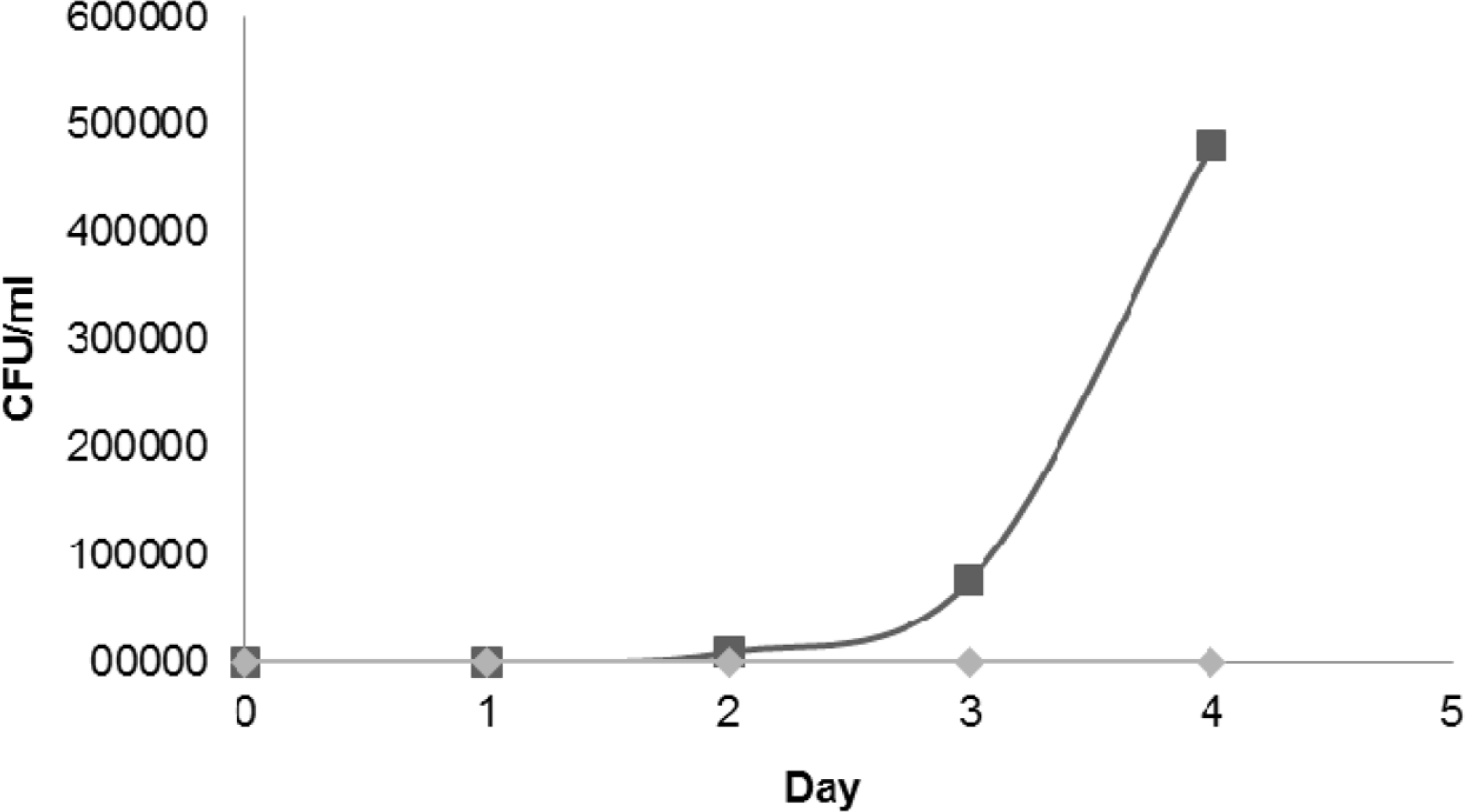
Table 1.
Antimicrobial activity of C. ternatea extract on disc diffusion assay. The extract showed antimicrobial activity on both Gram positive and Gram negative bacteria
Table 2.
The MIC and MLC of C. ternatea extract on tes microorganisms. The antimicrobial activity of the extract was con centration-dependent
| Bacteria | MIC (mg/ml) | MLC (mg/ml) |
|---|---|---|
| B. cereus | 25.0 | 50.0 |
| B. subtilis | 25.0 | 50.0 |
| S. aureus | 50.0 | 100.0 |
| P. mirabilis | 6.2 | 50.0 |
| K. pneumoniae | 1.6 | 25.0 |
| P. expansum | 12.5 | 25.0 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download