Abstract
Compositional differences in flavonoids are varied in the big family of Compositae. By summarizing our previous analytical studies and other scientific evidences, new strategy will be possible to further analyze flavonoids and utilize them for human health. The HPLC analytical method has been established in terms of linearity, sensitivity, accuracy, and precision. Herbs of the family of Compositae have considerable amounts of peroxynitrite (ONOO−)-scavenging effects and their phenolic substances. These effects may contribute to the prevention of disease associated with excess production of ONOO−, depending on the high content of flavonoid substances.
REFERENCES
(1). Fraisse D., Felgines C., Texier O., Lamaison J. L.Food Nutr. Sci. 2011; 2:181–192.
(2). Nugroho A., Kim K. H., Lee K. R., Alam M. B., Choi J. S., Kim W. B., Park H. J.Arch. Pharm. Res. 2009; 32:1361–1367.
(3). Nugroho A., Lee K. R., Alam M. B., Choi J. S., Park H. J.Arch. Pharm. Res. 2010; 33:703–708.
(4). Pacher P., Beckman J. S., Liaudet L.Physiol. Rev. 2007; 87:315–424.
(5). Nugroho A., Kim M. H., Lim S. C., Choi J. W., Choi J. S., Park H. J.Nat. Prod. Sci. 2011; 17:342–349.
(6). Nugroho A., Lim S. C., Lee C. M., Choi J. S., Park H. J. J.Pharm. Biomed. Anal. 2012; 61:247–251.
(7). Nugroho A., Kim M. H., Lee C. M., Choi J. S., Lee S. H., Park H. J.Nat. Prod. Sci. 2012; 18:39–46.
(8). Nugroho A., Lim S. C., Choi J. W., Park H. J.Arch. Pharm. Res. 2013; 36:51–60.
(9). Nugroho A., Lim S. C., Byeon J. S., Choi J. S., Park H. J. J.Pharm. Biomed. Anal. 2013; 76:139–144.
(10). Schmidt R. J.Clin. Dermatol. 1986; 4:46–61.
(11). Funk V. A., Susanna A., Stuessy T. F., Robinson H.Classifi-cation of Compositae. Systematics, evolution, and biogeography of the Compositae. Funk V.A., Susanna A., Bayer R. J., editorsInternational Association for Plant Taxonomy;Vienna: 2009. p. 174–189.
(12). Konarev A. V., Anisimova I. N., Gavrilova V. A., Vachrusheva T. E., Konechnaya G. Y., Lewis M., Shewry P. R.Phytochemistry. 2002; 59:279–291.
(13). Wegiera M., Smolarz H. D., Jedruch M., Korczak M., Kopro ní K.Acta Pol. Pharm. 2012; 69:263–268.
(14). Jan G., Khan M. A., Jan F.Ethnobot. Leaflets. 2009; 13:1205–1215.
(15). Ha T. J., Hwang S. W., Jung H. J., Park K. H., Yang M. S.Agric. Chem. Biotechnol. 2002; 45:170–172.
(16). Hitmi A., Barthomeuf C., Coudret A. J.Plant Phys. 1998; 153:233–236.
(18). Ksouri R., Megdiche W., Falleh H., Trabelsi N., Boulaaba M., Smaoui A., Abdelly C. C. R.Biol. 2008; 331:865–873.
(20). Youdim K. A., Shukitt-Hale B., Joseph J. A.Free Radic. Biol. Med. 2004; 37:1683–1693.
(21). Haenen G. R., Paquay J. B., Korthouwer R. E., Bast A.Biochem. Biophys. Res. Commun. 1997; 236:591–593.
(22). Korda M., Kubant R., Patton S., Malinski T.Am. J. Physiol. Heart Circ. Physiol. 2008; 295:1514–1521.
(23). Rayalam S., Della-Fera M. A., Baile C. A. J.Nutr. Biochem. 2008; 19:717–726.
(24). Tórtora V., Quijano C., Freeman B., Radi R., Castro L.Free Radic. Biol. Med. 2007; 42:1075–1088.
(25). Zhu S., Haddad I. Y., Matalon S.Arch. Biochem. Biophys. 1996; 333:282–290.
(26). Radi R., Beckman J. S., Bush K. M., Freeman B.A.J. Biol. Chem. 1991; 266:4244–4250.
(27). Tamura Y., Nakajima K., Nagayasu K., Takabayashi C.Phytochemistry. 2002; 59:275–278.
(28). Velíšek J., Davídek J., Cejpek K.Czech J. Food Sci. 2008; 26:73–98.
(29). Bowles D., Isayenkova J., Lim E. K., Poppenberger B.Curr. Opin. Plant Biol. 2005; 8:254–263.
(30). Agati G., Biricolti S., Guidi L., Ferrini F., Fini A., Tattini M. J.Plant Physiol. 2011; 168:204–212.
(31). Karki S., Park H. J., Nugroho A., Kim E. J., Jung H. A., Choi J. S. J.Med. Food. 2015; 18:83–94.
(32). Nugroho A., Choi J. S., An H. J., Park H. J.Nat. Prod. Sci. 2015; 21:42–48.
(33). Shimoi K., Okada H., Furugori M., Goda T., Takase S., Suzuki M., Hara Y., Yamamoto H., Kinae N.FEBS Lett. 1998; 438:220–224.
(34). Schneider H., Blaut M.Arch. Microbiol. 2000; 173:71–75.
(35). Lu J., Feng X., Sun Q., Lu H., Manabe M., Sugahara K., Ma D., Sagara Y., Kodama H.Clin. Chim. Acta. 2002; 316:95–99.
(36). Hu C., Kitts D. D.Mol. Cell. Biochem. 2004; 265:107–113.
(37). Jin M., Yang J. H., Lee E. K., Lu Y., Kwon S., Son K. H., Son J. K., Chang H. W.Biol. Pharm. Bull. 2009; 32:1500–1503.
(38). Qiusheng Z., Xiling S., Xubo X. S., Meng S., Changhai W.Pharmazie. 2004; 59:286–289.
(39). Vilela F. C., Soncini R., Giusti-Paiva A. J.Ethnopharmacol. 2009; 124:325–327.
(40). Freitas C. S., Baggio C. H., Finau J., Anginoni M., Pizzolatti M. G., Santos A. R., Marques M. C. J.Pharm. Pharmacol. 2008; 60:1105–1110.
(41). Kim J. S., Kwon C. S., Son K. H.Biosci. Biotechnol. Biochem. 2000; 64:2458–2461.
(42). Han X. H., Hong S. S., Hwang J. S., Lee M. K., Hwang B. Y., Ro J. S.Arch. Pharm. Res. 2007; 30:13–17.
(43). Brown J. E., Rice-Evans C. A.Free Radic. Res. 1998; 29:247–255.
(44). Min Y. S., Bai K. L., Yim S. H., Lee Y. J., Song H. J., Kim J. H., Ham I. H., Whang W. K., Sohn U. D.Arch. Pharm. Res. 2006; 29:484–489.
(45). Nagy M., Krizková L., Mucaji P., Kontseková Z., Sersen F., Krajcovic J.Molecules. 2009; 14:509–518.
(46). Vilela F. C., Padilha-Mde M., Alves-da-Silva G., Soncini R., Giusti-Paiva A. J.Med. Food. 2010; 13:219–222.
(47). Choi S. M., Kim B. C., Cho Y. H., Choi K. H., Chang J., Park M. S., Kim M. K., Cho K. H., Kim J. K.Chonnam Med. J. 2014; 50:45–51.
(48). Salqueiro J. B., Ardenghi P., Dias M., Ferreira M. B. C., Izquierdo I., Medina J. H.Pharmacol. Biochem. Behav. 1997; 58:887–891.
(49). Patil S. P., Jain P. D., Sancheti J. S., Ghumatkar P. J., Tambe R., Sathaye S.Neuropharmacolgy. 2014; 86:192–202.
(50). Ha S. K., Moon E., Lee P., Ryu J. H., Oh M. S., Kim S. Y.Neurochem. Res. 2012; 37:1560–1567.
(51). Watanabe K., Kanno S., Tomizawa A., Yomogida S., Ishikawa M.Oncol. Rep. 2012; 27:204–209.
(52). Fan S. Y., Zeng H. W., Pei Y. H., Li L., Ye J., Pan Y. X., Zhang J. G., Yuan X., Zhang W. D. J.Ethnopharmacol. 2012; 141:647–652.
(53). Calderone V., Chericoni S., Martinelli C., Testai L., Nardi A., Morelli I., Breschi M. C., Martinotti E.Naunyn Schmiedebergs Arch. Pharmacol. 2004; 370:290–298.
(54). Kim H. R., Park C. G., Jung J. Y.Int. J. Mol. Med. 2014; 33:317–324.
(55). Lim H., Son K. H., Chang H. W., Bae K., Kang S. S., Kim H. P.Biol. Pharm. Bull. 2008; 31:2063–2067.
(56). Yoo Y. M., Nam J. H., Kim M. Y., Choi J., Park H. J.Biol Pharm Bull. 2008; 31:760–764.
(57). Juckmeta T., Thongdeeying P., Itharat A.Evid. Based Complement. Alternat. Med. 2014; 2014:828760.
(58). Bors W., Heller W., Michael C., Saran M.Adv. Exp. Med. Biol. 1990; 264:165–170.
(59). Quiñones M., Miguel M., Aleixandre A.Pharmacol. Res. 2013; 68:125–131.
(60). Iwai K., Kishimoto N., Kakino Y., Mochida K., Fujita T. J.Agric. Food Chem. 2004; 52:4893–4898.
Fig. 1.
Characteristics of Compositae.
The head with ray florets arranged around the perimeter, disc florets in the center, and an involucre with bracts (phyllaries) surrounding the outermost florets. B. The pollen is released via the style pushing out through the anthers, which are fused at the margins; sometimes the style branches are recurved and come in contact with the style shaft. C. Some of the achene (cypsela) and pappus types found in Compositae (Funk et al., 2009).
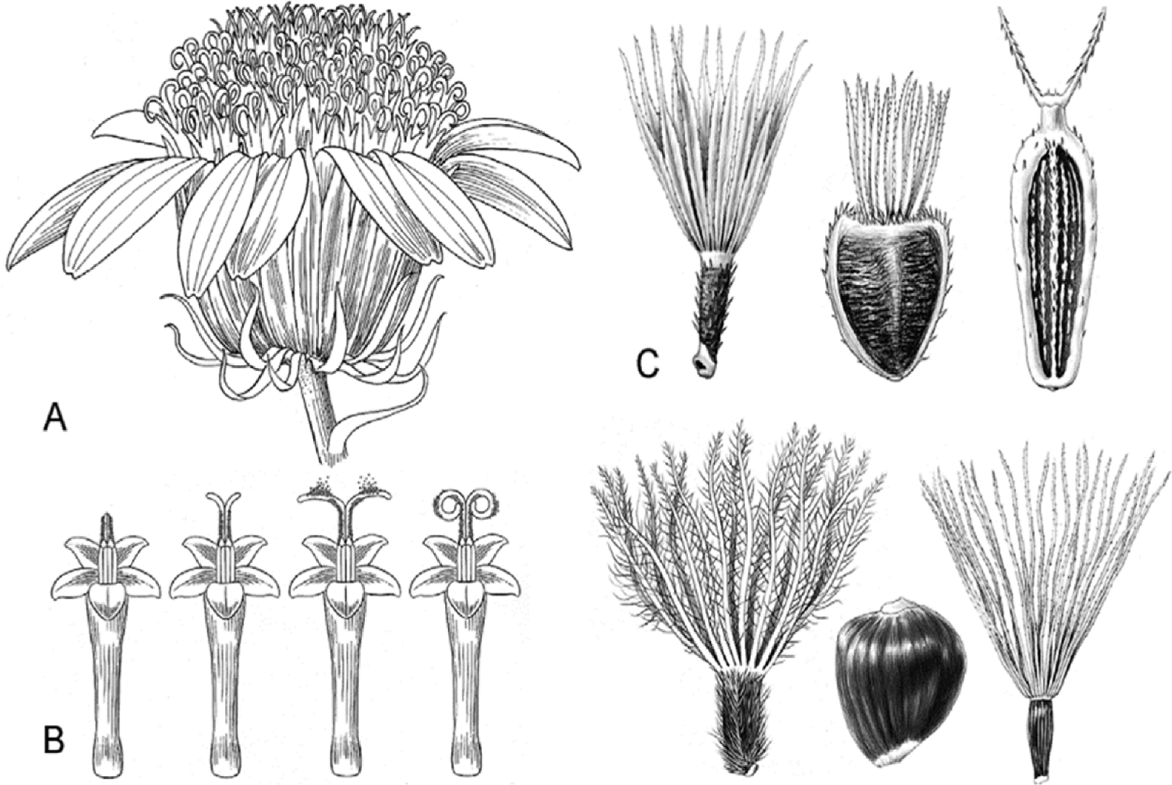
Fig. 4.
Structure of luteolin, acacetin, Lut-7-Glc, Lut-7-GlcU and acacetin identified from Y. japonica and their presumed pathway. A dotted arrow represents a less favored pathway.
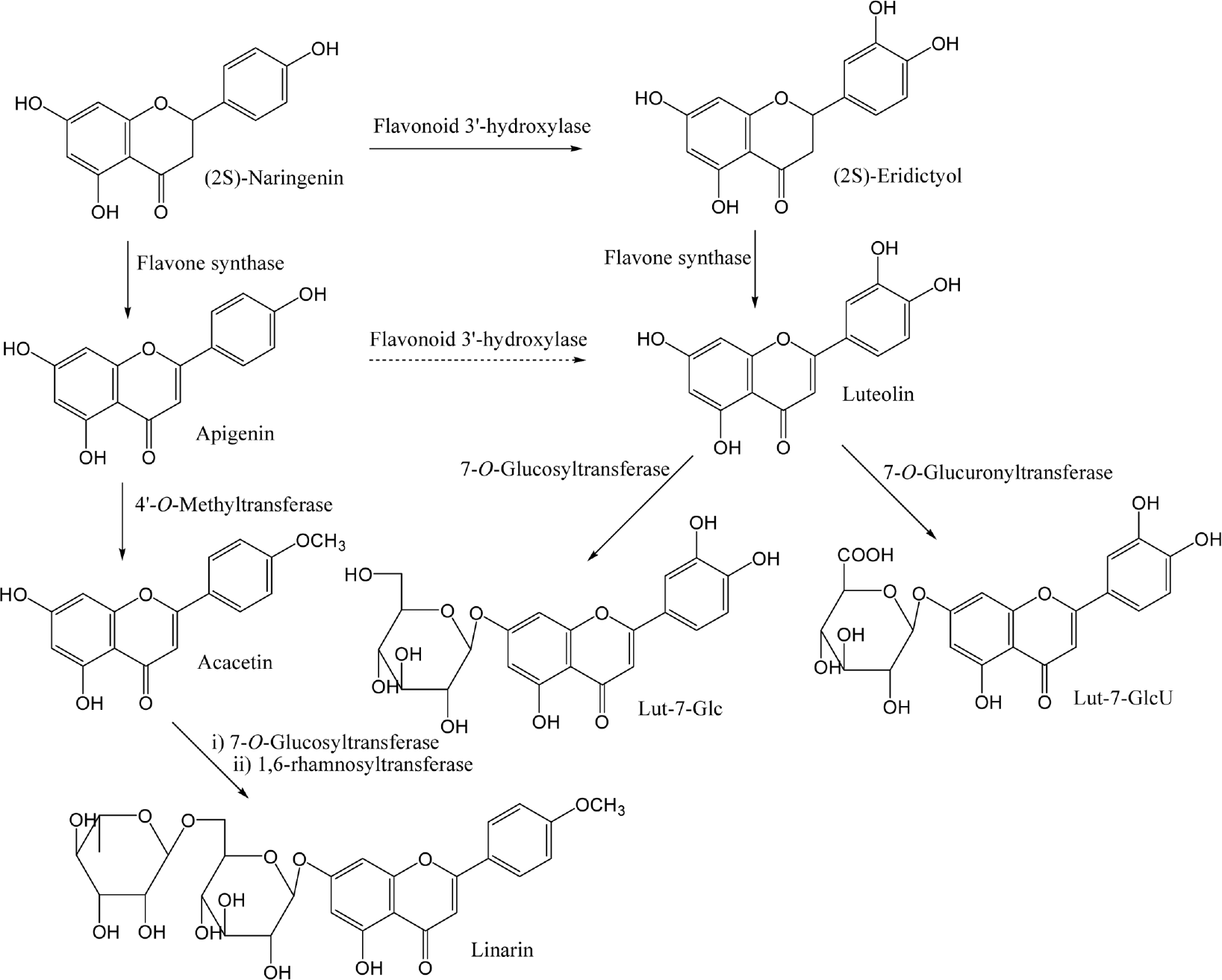
Fig. 5.
HPLC chromatograms of MeOH extracts of the four Compositae herbs (Compound names are abbreviated).
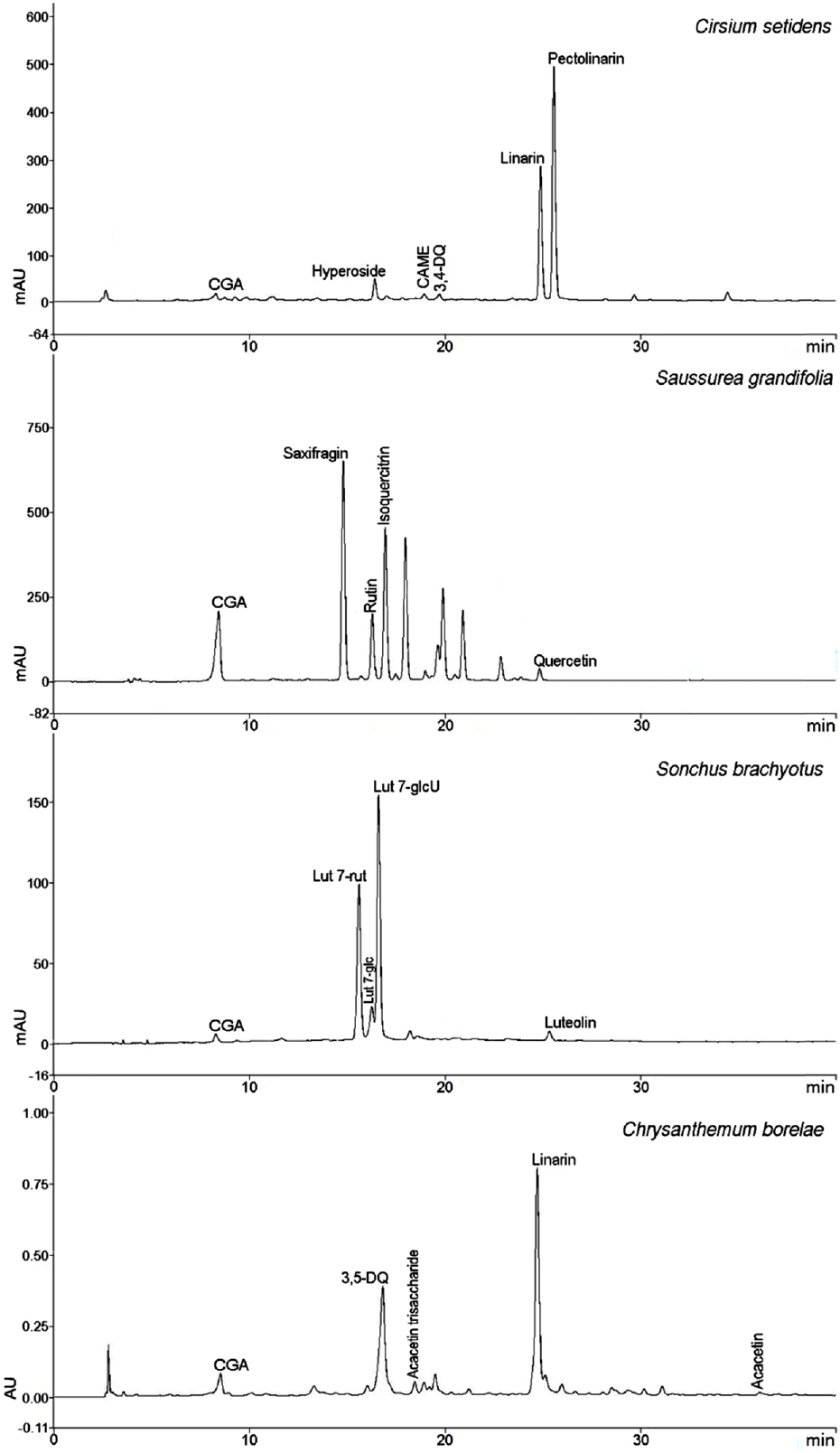
Fig. 7.
Peroxynitrite-scavenging activities (IC50 s) of the flavonoids and caffeoylquinic acids identified in the five Compositae herbs.
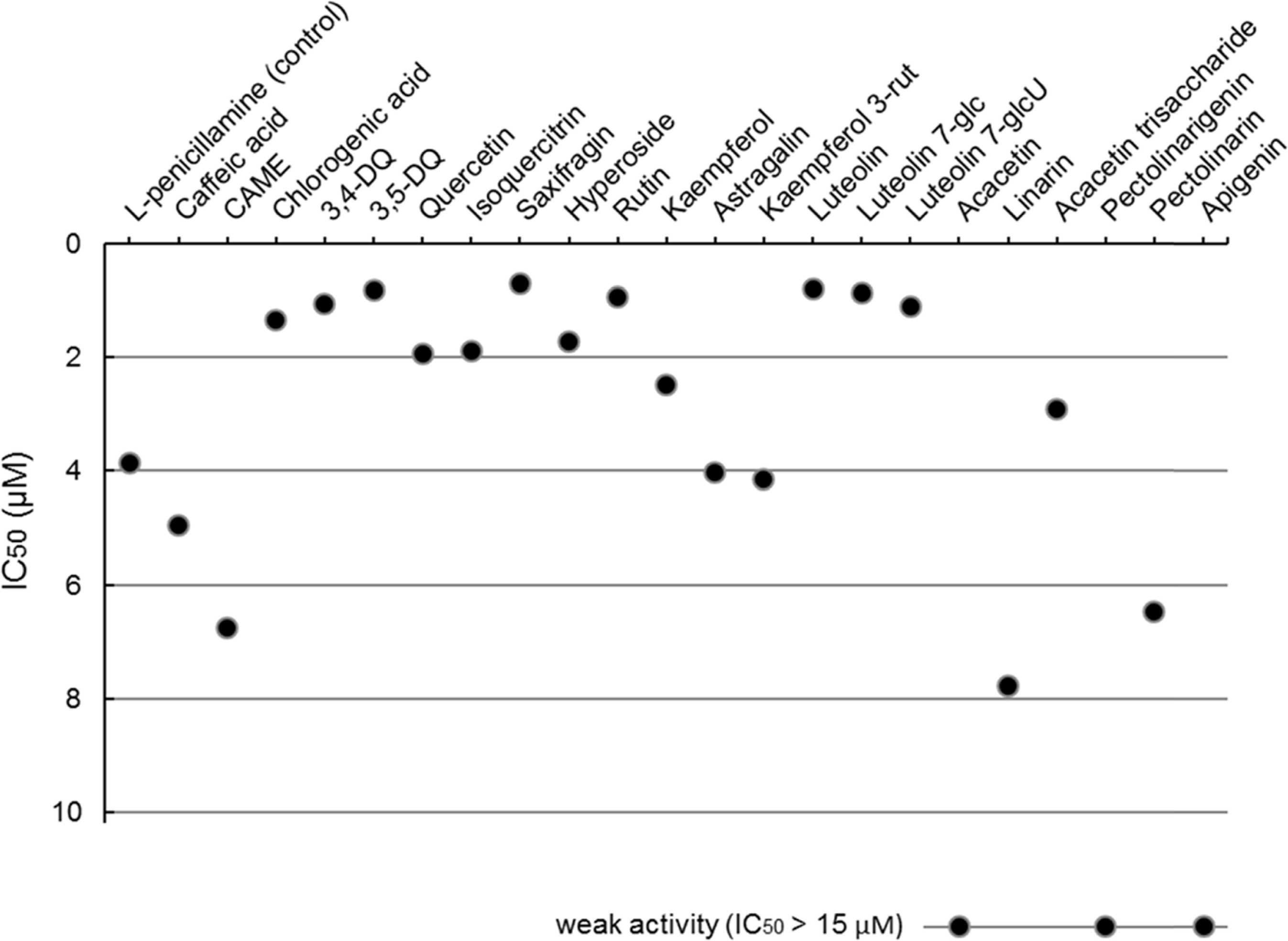
Table 1.
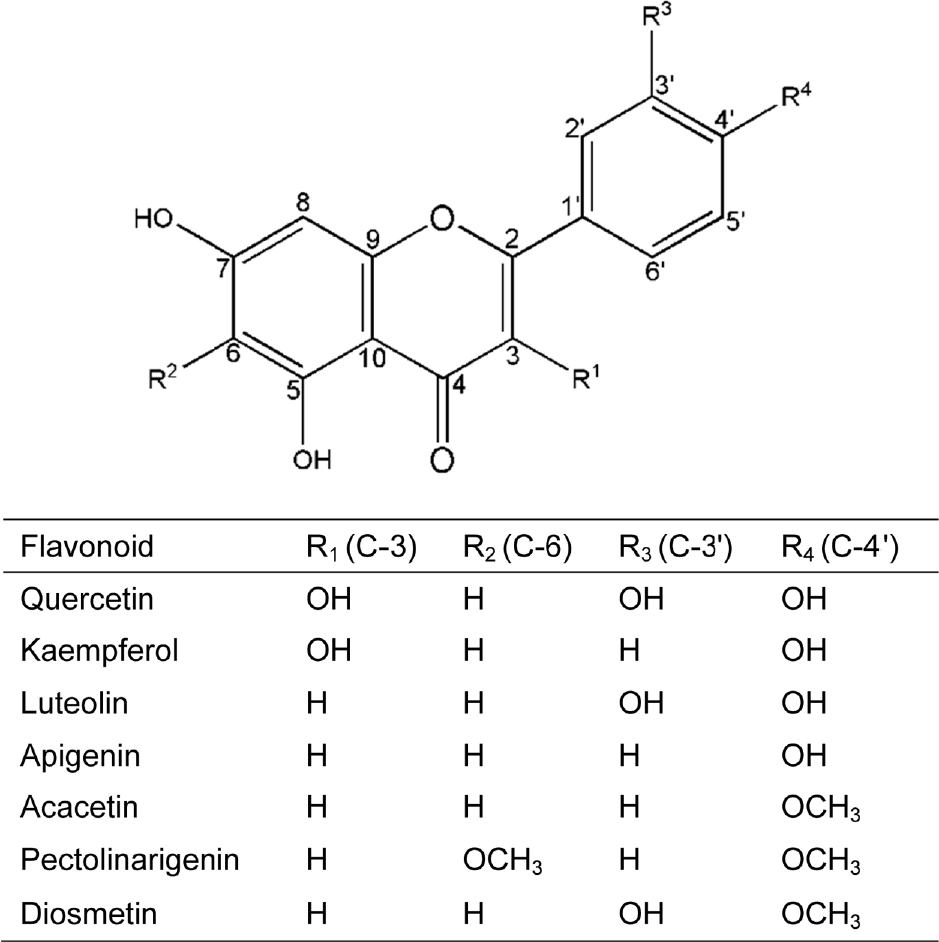
Recapitulation of the phenolics identified in the five Compositae herbs
Table 2.
Linearities and detection/quantification limits of the standard compounds
| Standard compound | tR (min) | Linear regressiona | R2b | LODc (µg/ml) | LOQd (µg/ml) |
|---|---|---|---|---|---|
| Chlorogenic acid | 8.32 | y = 166.80 x + 58.46 | 0.9998 | 0.30 | 1.01 |
| Caffeic acid | 10.36 | y = 253.42 x + 50.84 | 0.9999 | 0.22 | 0.72 |
| Saxifragin | 14.78 | y = 414.62 x + 65.93 | 0.9998 | 0.12 | 0.40 |
| Luteolin 7-rutinoside | 15.63 | y = 175.25 x + 34.55 | 0.9999 | 0.38 | 1.26 |
| Rutin | 16.13 | y = 143.53 x + 15.18 | 0.9999 | 0.42 | 1.39 |
| Luteolin 7-glucoside | 16.29 | y = 200.82 x + 40.73 | 0.9999 | 0.34 | 1.13 |
| Hyperoside | 16.37 | y = 217.04 x + 46.33 | 0.9999 | 0.14 | 0.46 |
| Luteolin 7-glucuronide | 16.63 | y = 48.813 x + 95.76 | 0.9997 | 0.85 | 2.83 |
| Isoquercitrin | 16.76 | y = 472.75 x + 75.62 | 0.9999 | 0.11 | 0.36 |
| 3,5-dicaffeoylquinic acid | 16.95 | y = 157.87 x + 75.02 | 0.9999 | 0.27 | 0.90 |
| Isorhoifolin | 18.09 | y = 232.85 x + 65.10 | 0.9999 | 0.17 | 0.57 |
| Acacetintrisaccharide | 18.42 | y = 104.76 x + 81.54 | 0.9999 | 0.30 | 1.01 |
| Kaempferol 3-rutinoside | 18.71 | y = 236.19 x + 54.25 | 0.9998 | 0.22 | 0.72 |
| 3,4-dicaffeoylquinic acid | 18.95 | y = 133.62 x + 45.50 | 0.9999 | 0.23 | 0.76 |
| Diosmin | 19.10 | y = 244.78 x + 53.54 | 0.9999 | 0.21 | 0.71 |
| Caffeic acid methyl ester | 19.68 | y = 172.39 x + 44.58 | 0.9999 | 0.12 | 0.40 |
| Quercetin | 24.79 | y = 659.28 x + 87.33 | 0.9999 | 0.09 | 0.23 |
| Linarin | 24.85 | y = 309.18 x + 30.86 | 0.9999 | 0.23 | 0.76 |
| Luteolin | 25.26 | y = 399.22 x + 164.9 | 0.9997 | 0.25 | 0.83 |
| Pectolinarin | 25.58 | y = 103.76 x + 60.10 | 0.9997 | 0.43 | 1.44 |
| Apigenin | 28.67 | y = 529.46 x + 49.28 | 0.9999 | 0.11 | 0.35 |
| Diosmetin | 29.53 | y = 741.76 x + 96.14 | 0.9999 | 0.01 | 0.04 |
| Acacetin | 36.88 | y = 369.27 x + 45.71 | 0.9999 | 0.15 | 0.50 |
| Pectolinarigenin | 37.73 | y = 199.06 x + 33.37 | 0.9999 | 0.20 | 0.67 |
Table 3.
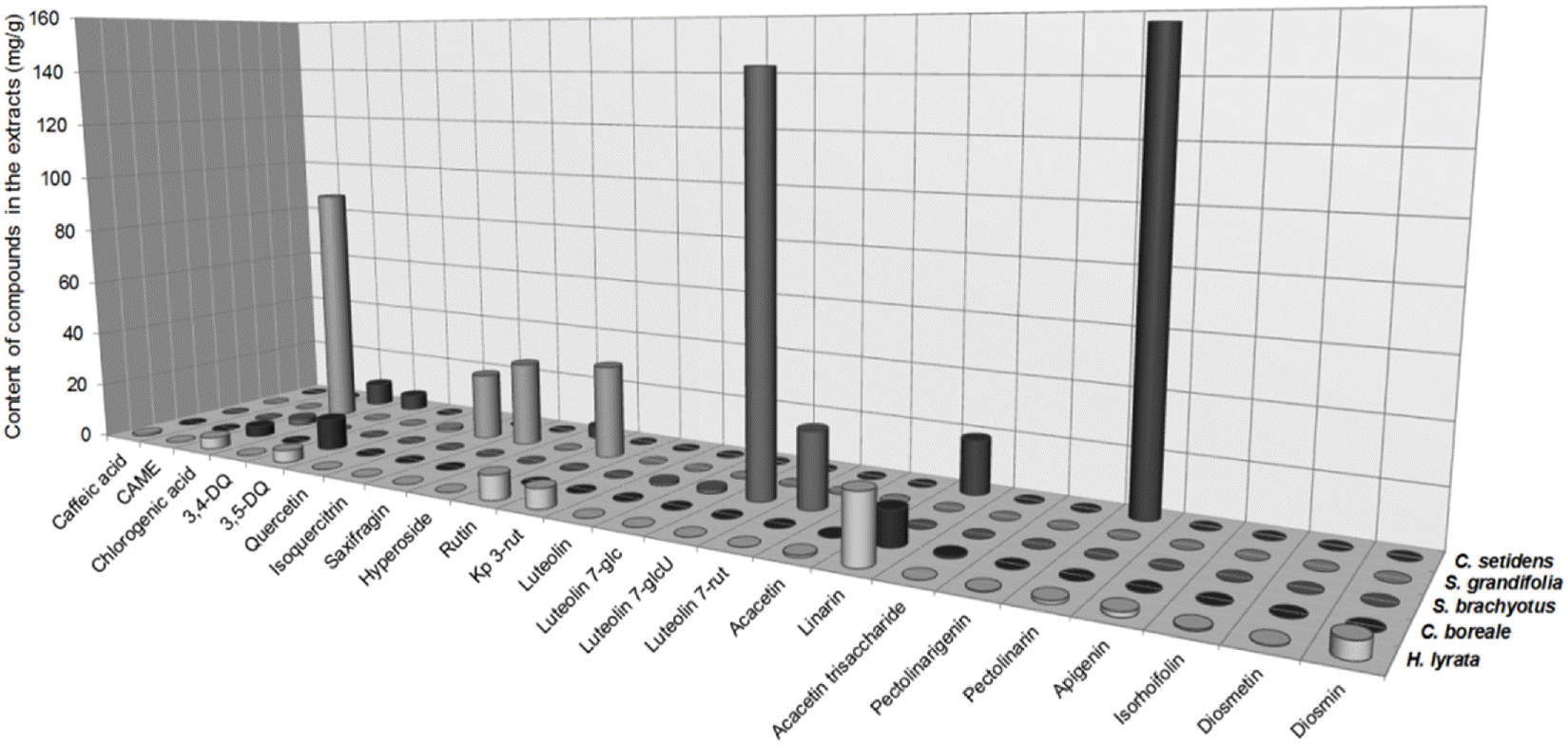
Contents of the flavonoids and caffeoylquinic acids in MeOH extracts of the five Compositae herbs (mg/g)
Table 4.
Contents of the flavonoids and caffeoylquinic acids in the dry plant material (DM) of the five Compositae herbs (mg/g of dry weight)
Table 5.
Peroxynitrite-scavenging activities (IC50 s) of the flavonoids and caffeoylquinic acids identified in the five Compositae herbs




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download