Abstract
Adding to the complexity of caring for critically ill patients is the fact that many of them have a creatinine clearance that exceeds 130 mL/min/1.73 m2. This phenomenon, termed augmented renal clearance (ARC), has only recently been widely recognized and its pathogenesis remains incompletely understood. However, ARC has been shown to result in increased dose requirements for drugs that are primarily eliminated by renal excretion, including many antimicrobial agents and enoxaparin. Recognition of ARC is hampered by the fact that the standard creatinine-based equations used to estimate renal function are not accurate in this clinical setting and the diagnosis is best established using both serum and urine creatinine measurements to calculate clearance. So a high index of clinical suspicion and awareness is usually required before this step is taken to confirm the diagnosis of ARC.
In 1974, Lucius Dettle proposed using creatinine clearance to estimate appropriate drug doses for patients with impaired renal function and a lowered glomerular filtration rate (GFR).[1] This likely represents the greatest clinical contribution that pharmacokinetics continues to make to the individualization of pharmacotherapy.[2] By contrast, attention has only recently been focused on the phenomenon of augmented renal clearance that in many critically ill patients counterintuitively accelerates drug elimination. This widespread but generally unappreciated phenomenon has led to its being referred to as “the elephant” in the intensive care unit.[3]
Although Zaske et al.[45] had previously noted that unusually high doses of aminoglycosides were required to attain therapeutic blood concentrations in burn patients. it was Loirat et al.[6] who in 1978 first attributed this to an increase in GFR. They observed that creatinine clearance in burn patients was as much as 200% higher than that of a comparator group of healthy subjects and confirmed, using radioiothalamate and inulin clearance measurements, that this reflected an increase in GFR. They found that the increase in creatinine clearance was most marked in the first two weeks after burn injury, that its magnitude was inversely correlated with patient age, and that it resulted in an increase in the rate of tobramycin elimination. The current consensus is that renal clearance should be considered augmented when creatinine clearance exceeds 130 ml/min/1.73 m2.[78] Other conditions predisposing to ARC include trauma other than burns,[910] infectious meningitis,[11] subarachnoid hemorrhage,[12] sepsis,[13] and other illness severe enough to merit intensive care unit admission.[14] Declerq et al.[15] have reported that ARC even was present in 30% of patients who had had abdominal surgery but did not require intensive care. Finally, GFR and renal clearance also are increased as part of the normal physiological adaptation to pregnancy.[16]
The incidence of ARC has been reported to range from 30% in patients after abdominal surgery[15] to 100% in patients with subarachnoid hemorrhage,[12] with an incidence of 55.8% in a general intensive care unit population.[14] Serum creatinine measurements have been within the normal range in all ARC patients.[8] So actual measurement of creatinine clearance is considered essential to making the diagnosis and both 8-hour[9] and 24-hour urine collections[67101112] have been used. Loirat et al.[6] found that creatinine clearance agreed closely with inulin and iothalamate clearance in this setting, and thus serves in these patients as a reliable measure of GFR. On the other hand, the serum creatinine-based equations that are routinely used to estimate renal function have been repeatedly shown to provide poor estimates of GFR in patients with ARC. [7171819]
Most studies have found that ARC is more common in younger patients who are less than 50 years of age than in patients who are older.[8] Udy et al.[13] have measured cardiac index in ARC patients with sepsis or trauma and have found it somewhat correlated with creatinine clearance in septic patients but not well correlated in trauma patients. A number of authors have examined the relationship between various severity of illness scores and the occurrence of ARC.[810] However, the identification of patients with ARC remains challenging and no satisfactory substitute has yet been found for actually measuring creatinine clearance.
Brown et al.[20] evaluated creatinine clearance and cardiac index in 50 surgical intensive care patients who had been admitted for trauma or nontraumatic surgical emergencies. Creatinine clearance was elevated in patients who did not develop renal failure and the extent of elevation was significantly correlated with cardiac index but ARC was not seen in patients who were more than 50 years old. These authors concluded that the increase in creatinine clearance represented part of a normal recovery response in younger patients. Udy et al.[21] extended these observations by using sinistrin and para-aminohippuric acid (PAH) as respective markers for GFR and effective renal plasma flow and demonstrated that both of these parameters were elevated in patients with ARC. Although both tubular anion secretion of PAH and tubular reabsorption of fluconazole were increased, tubular cationic secretion of pindolol was reduced. Udy[22] also reported that changes in creatinine clearance generally paralleled changes in cardiac output in ARC patients.
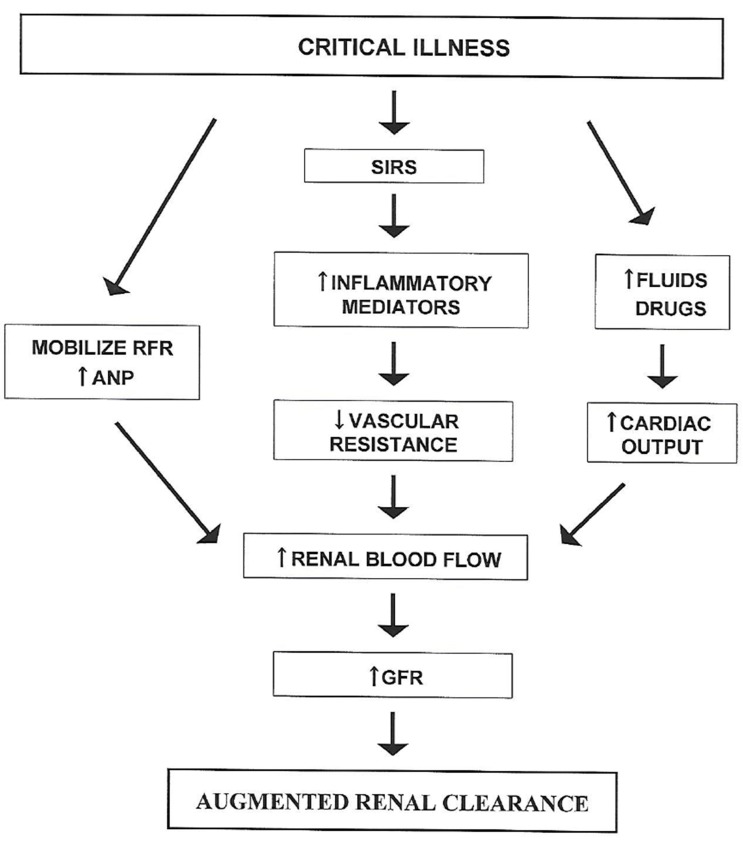
Both cardiac output and GFR increase as part of the normal physiological response to pregnancy.[23] However, the increase in cardiac output occurs subsequent to the initial increase in GFR which results from widespread peripheral vasodilation. A similar sequence of events in other settings of ARC has been attributed to the systemic inflammatory response syndrome (SIRS).[24] Although the concept of SIRS was initially developed to help characterize patients with sepsis, it has subsequently been found to be equally applicable to patients with burns and other trauma in which an initial injury leads to the subsequent release of multiple cytokine mediators of inflammation that cause widespread vasodilation.[25] Further stimuli that could increase cardiac output in critically ill patients include administration of fluids and drugs such as dopamine.[24] This concept of pathophysiology has led to the schema shown in Figure 1.
Udy et al.[22] found that atrial natriuretic peptide (ANP) plasma concentrations were also increased in patients with traumatic brain injury. They speculated that this could be contributing to the ARC observed in brain injury patients since ANP is known to increase both GFR and renal sodium excretion.[26] However, sequential measurements of creatinine clearance and ANP concentrations in individual patients indicated that there was an inverse relationship between changes in creatinine clearance and ANP concentrations. They concluded that intravascular volume and atrial wall stretch decreased as GFR increased, thus causing ANP secretion to be decreased.
Activation of renal functional reserve has also been postulated to contribute to ARC.[21] The existence of renal functional reserve was first identified by Shannon, Jolliffe, and Smith[27] who noted a marked increase in GFR after dogs were fed a high protein meal. This phenomenon has since been studied extensively and Molitoris[28] feels that basal GFR measurements do not adequately characterize kidney function because they do not take renal functional reserve into account. Although it has been demonstrated in pregnant women that renal functional reserve is normal, and thus has not been activated by pregnancy itself,[16] similar studies have not been conducted in other categories of patients with ARC. So this remains one of the factors that may be contributing, probably in combination with other factors, to what appears to be a fairly general response to injury.
ARC is generally considered to be a favorable marker of a host's ability to respond to injury.[8] So the main concern is that the increased renal function will lead to suboptimal therapy with drugs that are primarily eliminated by renal excretion. In fact, the need for burn patients to receive increased doses of gentamicin[4] and amikacin[5] was noted even before Loirat et al.[6] reported that an increase in GFR required increased tobramycin doses in this clinical setting. The clinical impact of ARC has largely been reported in the context of antimicrobial therapy, not only with aminoglycosides, but also with vancomycin[29] and β-lactams.[30] In addition, patients with ARC have also been found to have a shorter duration of anticoagulant effect when standard doses of enoxaparin were administered.[31]
Although it seems intuitively obvious that ARC should lead to an increased rate of treatment failure unless doses of renally excreted drugs are appropriately increased, conflicting results have been reported in the few outcome studies that have been conducted. An initial study reported by Claus et al.[32] did find that antibiotic failures were more common in critically ill patients with ARC than in those with lower measured creatinine clearance. Thus, therapeutic failure was present for 33.3% of patients with ARC that lasted for more than one day, for 17.4% of patients with transient ARC that lasted for only one day but for just 12.9% of patients without ARC. The authors excluded aminoglycosides, teicoplanin, and vancomycin because therapeutic drug monitoring (TDM) was used to adjust doses of these antibiotics, but TDM was not used for the β-lactams, quinolone, and other antibiotics that were included in the study.
More recent outcome studies have focused solely on β-lactam antibiotics and have yielded conflicting results. Hutter et al.[33] studied 100 critically ill patients with clinically suspected or microbiologically proven severe bacterial infections. TDM was employed and intermediate and trough antibiotic concentrations were compared with the non-species-related breakpoints published by the European Committee on Antimicrobial Susceptibility Testing (EUCAST).[34] Standard antibiotic doses were administered and patients with ARC at study inclusion were found to be 3.3 times more likely to have at least one undetectable β-lactam trough concentration (<0.2 µg/mL). However neither the presence of ARC nor extremely low trough β-lactam concentrations were significantly associated with clinical failure. The authors reasoned that their use of antibiotics in addition to β-lactams and the fact that organism minimum inhibitory concentrations (MICs) were not measured, and might have been less than 0.2 µg/mL, both might have contributed to these surprising results. Further confounding the analysis of their results is the fact that the underlying prognosis of patients with ARC is generally more favorable that than of patients with lower creatinine clearances.
Udy et al.,[35] in an analysis of 254 patients with severe sepsis, also found that ARC did not compromise the efficacy of either intermittent or continuous infusion of β-lactam antibiotics. In fact, clinical cure, defined by patient response observed 14 days after stopping antibiotic therapy, was more common in ARC patients than in those with lower creatinine clearances. Unfortunately, interpretation of these results is clouded by the facts that antibiotic doses, antibiotic plasma concentrations, or susceptibility of the infecting organisms to the administered antibiotics were not reported.
Carrie[30] recently reported a study of 79 critically ill patients with sepsis but without renal failure who were treated with continuous intravenous infusions of standard doses of β-lactam antibiotics. The MIC of the infecting organisms was either measured or estimated from the EUCAST clinical breakpoints. Non-protein bound (free) antibiotic concentrations were measured during the first three days of therapy. Although 44 of the 79 patients had a creatinine clearance ≥ 150 mL/min, none of the patients had measured antibiotic concentrations below the MIC of the known pathogen. On the other hand, taking free concentrations of ≥ 4 times the MIC as a target for maximal efficacy, patients with creatinine clearance values ≥ 170 mL/min were statistically more likely to be under dosed, with a β-lactam subexposure rate of 20%. Those patients with β-lactam subexposure had a higher rate of secondary acquisition of β-lactam resistance and therapeutic failure. The interpretation of these results is again complicated by the frequent administration of combination antibiotic therapy and the underlying better prognosis of ARC patients. In addition to MIC measurements, some indication of bacterial load as well as TDM should probably also be incorporated in future studies.[36]
The presumption is that ARC may adversely impact the effectiveness of any drugs that depend primarily on renal excretion for their elimination. Udy et al.[37] have pointed out that “you only find what you look for” and have emphasized that the finding of a normal serum creatinine concentration does not equate to normal renal function in the setting of ARC. So a high index of suspicion needs to be maintained if ARC is to be diagnosed so that critically ill patients can be treated appropriately. Once ARC is diagnosed, TDM, if available, might be helpful in treating these patients with a number of drugs that are primarily eliminated by renal excretion.
References
1. Dettli L. Individualization of drug dosage in patients with renal disease. Med Clin North Am. 1974; 58:977–985. PMID: 4425338.

2. Atkinson AJ Jr, Huang SM. Nephropharmacology: Drugs and the kidney. Clin Pharmacol Ther. 2009; 86:453–456. DOI: 10.1038/clpt.2009.191. PMID: 19844216.

3. Baptista JP, Udy AA. Augmented renal clearance in critical illness: “The Elephant in the ICU”. Minerva Anestesiol. 2015; 81:1050–1052. PMID: 25881734.
4. Zaske DE, Sawchuk RJ, Gerding DN, Strate RG. Increased dosage requirements of gentamicin in burn patients. J Trauma. 1976; 16:824–828. PMID: 994263.

5. Zaske DE, Sawchuk RJ, Strate RG. The necessity of increased doses of amikacin in burn patients. Surgery. 1978; 84:603–608. PMID: 715674.
6. Loirat P, Rohan J, Baillet A, Beaufils F, David R, Chapman A. Increased glomerular filtration rate in patients with major burns and its effect on the pharmacokinetics of tobramycin. N Engl J Med. 1978; 299:915–919. PMID: 692596.

7. Ruiz S, Minville V, Asehnoune K, Virtos M, Georges B, Fourcade O, et al. Screening of patients with augmented renal clearance in ICU: taking into account the CKD-EPI equation, the age, and the cause of admission. Ann Intensive Care. 2015; 5:49. DOI: 10.1186/s13613-015-0090-8. PMID: 26667819.

8. Bilbao-Meseguer I, Rodriguez-Gascón A, Rarrasa H, Isla A, Solinís MÁ. Augmented renal clearance in critically ill patients: A systematic review. Clin Pharmacokinet. 2018; 57:1107–1121. DOI: 10.1007/s40262-018-0636-7. PMID: 29441476.

9. Udy AA, Jarrett P, Lassig-Smith M, Stuart J, Starr T, Dunlop R, et al. Augmented renal clearance in traumatic brain injury: a single-center observational study of atrial natriuretic peptide, cardiac output, and creatinine clearance. J Neurotrauma. 2017; 34:137–144. DOI: 10.1089/neu.2015.4328. PMID: 27302851.
10. Barletta JF, Mangram AJ, Byrne M, Sucher JF, Hollingworth AK, Ali-Osman FR, et al. Identifying augmented renal clearance in trauma patients: Validation of the augmented renal clearance in trauma intensive care scoring system. J Trauma Acute Care Surg. 2017; 82:665–671. DOI: 10.1097/TA.0000000000001387. PMID: 28129261.
11. Lautrette A, Phan TN, Ouchchane L, AitiHassan A, Tixier V, Heng AE, et al. High creatinine clearance in critically ill patients with community-acquired acute infectious meningitis. BMC Nephrol. 2012; 13:124. DOI: 10.1186/1471-2369-13-124. PMID: 23013403.

12. May CC, Arora S, Parli SE, Fraser JF, Bastin MT, Cook AM. Augmented renal clearance in patients with subarachnoid hemorrhage. Neurocrit Care. 2015; 23:374–379. DOI: 10.1007/s12028-015-0127-8. PMID: 25761425.

13. Udy AA, Roberts JA, Shorr AF, Boots RJ, Lipman J. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: identifying at-risk patients. Crit Care. 2013; 17:R35. DOI: 10.1186/cc12544. PMID: 23448570.

14. De Waele JJ, Dumoulin A, Janssen A, Hoste EA. Epidemiology of augmented renal clearance in mixed ICU patients. Minerva Anestesiol. 2015; 81:1079–1085. PMID: 25697881.
15. Declercq P, Nijs S, D'Hoore A, Van Wijngaerden E, Wolthuis A, de Buck van Overstraeten A, et al. Augmented renal clearance in non-critically ill abdominal and trauma surgery patients is an underestimated phenomenon: a point prevalence study. J Trauma Acute Care Surg. 2016; 81:468–477. DOI: 10.1097/TA.0000000000001138. PMID: 27257707.
16. Sturgiss SN, Wilkinson R, Davison JM. Renal reserve during human pregnancy. Am J Physiol. 1996; 271:F16–F20. PMID: 8760238.

17. Baptista JP, Udy AA, Sousa E, Pimentel J, Wang L, Roberts JA, et al. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit Care. 2011; 15:R139. DOI: 10.1186/cc10262. PMID: 21651804.

18. Grootaert V, Willems L, Debaveye Y, Meyfroidt G, Spriet I. Augmented renal clearance in the critically ill: how to assess kidney function. Ann Pharmacother. 2012; 46:952–959. DOI: 10.1345/aph.1Q708. PMID: 22693271.

19. Tsai D, Udy AA, Stewart PC, Gourley S, Morick NM, Lipman J, et al. Prevalence of augmented renal clearance and performance of glomerular filtration estimates in indigenous Australian patients requiring intensive care admission. Anaesth Intensive Care. 2018; 46:42–50. PMID: 29361255.

20. Brown R, Babcock R, Talbert J, Gruenberg J, Czurak C, Campbell M. Renal function in critically ill postoperative patients: sequential assessment of creatinine osmolar and free water clearance. Crit Care Med. 1980; 8:68–72. PMID: 7353390.
21. Udy A, Jarrett P, Stuart J, Lassig-Smith M, Starr T, Dunlop R, et al. Determining the mechanisms underlying augmented renal drug clearance in the critically ill: use of exogenous marker compounds. Crit Care. 2014; 18:657. DOI: 10.1186/s13054-014-0667-z. PMID: 25432141.

22. Udy AA, Jarrett P, Lassig-Smith M, Stuart J, Starr T, Dunlop R, et al. Augmented renal clearance in traumatic brain injury: a single-center observational study of atrial natriuretic peptide, cardiac output, and creatinine clearance. J Neurotrauma. 2017; 34:137–144. DOI: 10.1089/neu.2015.4328. PMID: 27302851.

23. Varga I, Rigó J Jr, Somos P, Joó JG, Nagy B. Analysis of maternal circulation and renal function in physiologic pregnancies: parallel examinations of the changes in the cardiac output and the glomerular filtration rate. J Matern Fetal Med. 2000; 9:97–104. PMID: 10902822.

24. Sime FB, Udy AA, Roberts JA. Augmented renal clearance in critically ill patients: etiology, definition and implications for beta-lactam dose optimization. Curr Opin Pharmacol. 2015; 24:1–6. DOI: 10.1016/j.coph.2015.06.002. PMID: 26119486.

25. Bone RC. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: What we do and do not know about cytokine regulation. Crit Care Med. 1996; 24:163–172. PMID: 8565523.
26. Song W, Wang H, Wu Q. Atrial natriuretic peptide in cardiovascular biology and disease (NPPA). Gene. 2015; 569:1–6. DOI: 10.1016/j.gene.2015.06.029. PMID: 26074089.
27. Shannon JA, Jolliffe N, Smith HW. The excretion of urine in the dog. IV. The effect of maintenance diet, feeding, etc., upon the quantity of glomerular filtrate. Am J Physiol. 1932; 101:625–638.
28. Molitoris BA. Rethinking CKD evaluation: should we be quantifying basal or stimulated GFR to maximize precision and sensitivity? Am J Kidney Dis. 2017; 69:675–683. DOI: 10.1053/j.ajkd.2016.11.028. PMID: 28223001.
29. Baptista JP, Sousa E, Martins PJ, Pimentel JM. Augmented renal clearance in septic patients and implications for vancomycin optimization. Int J Antimicrob Agents. 2012; 39:420–423. DOI: 10.1016/j.ijantimicag.2011.12.011. PMID: 22386742.
30. Carrié C, Petit L, d'Houdain N, Sauvage N, Cottenceau V, Lafitte M, et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of β-lactams administered by continuous infusion: a prospective observational study. Int J Antimicrob Agents. 2018; 51:443–449. DOI: 10.1016/j.ijantimicag.2017.11.013. PMID: 29180280.

31. Abdel El, Abdelhamid MHE, Atteya DAM. Impact of augmented renal clearance on enoxaparin therapy in critically ill patients. Egypt J Anesth. 2017; 33:113–117.
32. Claus BO, Hoste EA, Colpaert K, Robays H, Decruyenaere J, De Waele JJ. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care. 2013; 28:695–700. DOI: 10.1016/j.jcrc.2013.03.003. PMID: 23683557.

33. Huttner A, Von Dach E, Renzoni A, Huttner BD, Affaticati M, Pagani L, et al. Augmented renal clearance, low β-lactam concentrations and clinical outcomes in the critically ill: An observational prospective cohort study. Int J Antimicrob Agents. 2015; 45:385–392. DOI: 10.1016/j.ijantimicag.2014.12.017. PMID: 25656151.

34. European Commission on Antimicrobial Susceptibility Testing. Accessed 17 May 2018. Internet at: http://www.eucast.org/clinical.breakpoints/.
35. Udy AA, Dulhunty JM, Roberts JA, Davis JS, Web SAR, Bellomo R, et al. Association between augmented renal clearance and clinical outcomes in patients receiving β-lactam antibiotic therapy by continuous or intermittent infusion: a nested cohort study of the BLING-II randomized placebo-controlled, clinical trial. Int J Antimicrob Agents. 2017; 49:624–630. DOI: 10.1016/j.ijantimicag.2016.12.022. PMID: 28286115.
36. Udy AA, De Waele JJ, Lipman J. Augmented renal clearance and therapeutic monitoring of β-lactams. Int J Antimicrob Agents. 2015; 45:331–333. DOI: 10.1016/j.ijantimicag.2014.12.020. PMID: 25665727.

37. Udy A, Roberts JA, Boots RJ, Lipman J. You only find what you look for: the importance of high creatinine clearance in the critically ill. Anaesth Intensive Care. 2009; 37:11–13. PMID: 19157339.

Figure 1
Current understanding of the pathophysiology of ARC in which critical illness triggers the systemic inflammatory response syndrome (SIRS) or mobilizes renal functional reserve (RFR) to eventually increase renal blood flow and glomerular filtration rate (GFR). Intravenous fluids and drugs administered to critically ill patients may increase cardiac output and contribute to the increase in renal blood flow. Atrial natriuretic peptide (ANP) plasma concentrations are also elevated in patients with ARC and may play a role in increasing renal blood flow. (Modified from Sime FB, Udy AA, Roberts JA. Curr Opin Pharmacol 2015;24:1-6.)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download