Abstract
A clinical trial management system (CTMS) is a comprehensive program that supports an efficient clinical trial. To improve the environment of clinical trials and to be competitive in the global clinical trials market, an advanced and integrated CTMS is necessary. However, there is little information about the status of CTMSs in Korea. To understand the utilization of current CTMSs and requirements for a future CTMS, we conducted a survey on the subjects related to clinical trials. The survey was conducted from July 27 to August 16, 2017. The total number of respondents was 596, and 531 of these responses were used. Almost half of the respondents were from hospitals (46%). The proportion of respondents who are currently using a CTMS was the highest for contract research organizations at 59%, whereas the proportion used by investigators was 39%. The main reason for not using a CTMS was that it is unnecessary and expensive, but it showed a difference between workplaces. Many respondents frequently used CTMSs to check the clinical trial schedule and progress status, which was needed regardless of workplace. While two-thirds of users tended to be satisfied with their current CTMS, there were many users who felt their CTMS was inconvenient. The most requested function for a future CTMS was one that could be used to manage the project schedule and subject enrollment status. Additionally, a systematic linkage to electronic medical records, including prescription and laboratory test results, and a function to confirm the participation history of subjects in other hospitals were requested.
A clinical trial is an investigation in human subjects intended to discover or verify the clinical, pharmacological, or pharmacodynamic effects, or identify adverse reactions, or study pharmacokinetic effects of an investigational product.[1] Therefore, the conduct of clinical trials is an important part of the development of new drugs.
In Korea, the number of clinical trials was less than 100 in the early 2000s. As the pharmaceutical industry became global, the Korean government recognized that clinical trials are a high value-added industry. Since then, Korea has dramatically grown and maintained its position in the global clinical trial market, with positive support from the government and excellent professional manpower.[23] However, costs for clinical trials in Korea have become expensive, whereas newly developing countries, such as China, India, and Brazil, still have lower costs and have the advantage of large populations.[45] Furthermore, the number of global sponsor-initiated clinical trials are decreasing due to the slowdown in pharmaceutical market growth, leading to rapid changes in the global clinical trial market and increased competition in attracting global clinical trials.[2] Therefore, the Korean government announced plans involving the development of an integrated clinical trial management system (CTMS) that can strengthen the competitiveness in the intense clinical trial industry.[5]
A CTMS is a comprehensive system for the management of clinical trials that can help to effectively manage clinical trials and provide oversight of clinical trials. A CTMS can include functions to recruit subjects, record case report forms (CRFs), manage the overall project schedules, enter the results data, conduct statistical analyses, and monitor the conduct of the clinical trial. The features of a CTMS are still expanding. Oracle, one of the major clinical solution companies, regards the CTMS as a central hub of other clinical trial-related systems and proposed that the CTMS should drive a convergence of such systems, thus forming a clinical trial ecosystem.[6] Recently, the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) revised the Good Clinical Practice (GCP) guidelines to emphasize the role of sponsors in data management and to monitor the management of essential documents, including source documents.[1] This revision creates an increased need and demand for CTMSs to manage multinational and multicenter clinical trials efficiently.
Institutes and organizations in Korea also use CTMSs to conduct clinical trials. Some large-scale institutes, such as Seoul National University Hospital and Asan Medical Center, developed CTMSs themselves, while relatively small-scale institutes or individual investigators have purchased commercial CTMS solutions or have used systems managed by government organizations.[7] As a result, the utilization of CTMSs has been limited to the management of in-house clinical trials and the associated data. However, clinical trials have become more complex with the globalization of the pharmaceutical industry, and the current global trend involves integrated, systematic environments that can manage clinical trials effectively.
To improve the general environment of clinical trial institutions and to be more competitive in the global clinical trial industry, the development of an integrated CTMS is needed. This research was the first step of a plan to investigate the status of current CTMS usage and plans for a future CTMS, and a survey was conducted to investigate potential CTMS users' current thoughts and needs.
In this research, an online survey platform named Moaform (www.moaform.co.kr) was used to generate questionnaires, and a link to our questionnaire was distributed. The target respondents we wanted to reach were those who were working in fields related to clinical trials such as hospitals, contract research organizations (CROs), pharmaceutical companies, and related information technology (IT) companies. The response period was from July 27 to August 16, 2017.
A questionnaire of this research was developed on our own method to understand the status of usage of CTMS and the demands of potential users on the future CTMS. The questionnaire consisted of a total nine questions in three categories of the characteristics of respondents, their usage of CTMS, and the requirement for the future CTMS. The respondent's workplace and the duration of their clinical trials-related career were required to understand the overall distribution of respondents. Whether or not they were using CTMS and, if not, the reason were also asked. For current and former users, we asked for the name of the CTMS program they used, the most frequently used function, the respondent's satisfaction with the current CTMS, and the reason for their satisfaction. The name of the CTMS program and the reasons for satisfaction were open and optional questions. Every respondent was required to choose three functions they thought should be included in a future CTMS out of 13 closed answers. The items mentioned in the questionnaire were related to managing subjects and clinical trials and performing clinical trials; they also included system-connective functions, communication functions, and other helpful functions. Other useful functions that should be included in a future CTMS but were not mentioned within the list of examples; instead, they were collected using an open and optional question.
After receiving categorical responses for all of the questions in the questionnaire, frequency analysis for the answers was performed. For closed questions, every answer was separately analyzed for the respondent's workplace, and a frequency table was created. Chi-square tests were conducted for the results to examine whether there were differences. Additionally, the reasons given for not using CTMSs were separately analyzed for the user's previous experience with CTMSs, and the results were expressed as a bar chart with percentages. All analyses were conducted using R 3.5.0 (R Development Core Team, 2017), and p-values < 0.05 were regarded as statistically significant.
The total number of respondents in the survey was 596. There were two duplicate respondents and 63 invalid responses, including 48 contradictory responses about the function they used and the satisfaction (e.g., Never used / Somewhat satisfied), and 15 respondents who had not used a CTMS while it was used in their workplace. Therefore, 531 responses were used for our analyses.
As shown in Table 1, almost half of the respondents were working at hospitals (46%), and most respondents had a work experience of less than 10 years (85%). However, there were some differences between workplace groups (p < 0.001), where respondents from CROs and IT-related companies had relatively short work experience.
In Table 2, the results of usage status of the CTMS were presented. The percentage of respondents who were currently using CTMSs was 43%, and 16% of the respondents were considered to have previously used CTMSs from the results of three questions (Q3, Q4, and Q5). The user group with the largest percentage of CTMS users was CRO employees, and there were no current users in IT-related companies. For hospitals, approximately 4 out of 10 respondents were currently using CTMSs (39%), and 45% of respondents had never used CTMSs (Fig. 1).
The respondents who were not currently using CTMSs were inquired about the reason for their lack of utilization. The most common reason given for not using a CTMS was that because it was not necessary (45%). However, there was a difference between workplace groups. More respondents at hospitals and pharmaceutical companies answered that they did not use a CTMS because of the high cost, unlike respondents at CROs, IT-related companies, and other workplaces. Additionally, there was a difference among respondents who had previously used a CTMS and those who had never used one. While respondents who had never used a CTMS mostly responded that CTMS usage was not necessary (52%), respondents who had previously used a CTMS responded they were not using a CTMS because of its high cost (55%), with a significantly fewer respondents answering that a CTMS was unnecessary (26%) (Fig. 2).
The current and previous users were asked about a function they used most frequently and their satisfaction with the current CTMS (Q5). A function to check the clinical trial schedule and progress status was most frequently used (40%), but the answers differed according to where the respondents worked (p < 0.001). In the case of hospitals, a function that supported entering clinical trial results data was selected the most often (32%), but in the case of CROs and pharmaceutical companies, the majority of users used a function to check the clinical trial schedule and progress status (69% and 49%, respectively).
Approximately two-thirds of users positively answered for their experience with CTMSs (Q6). However, more than 2 out of 10 users were not satisfied with their current CTMS (21%), and 4 out of 10 respondents had never used a CTMS (41%) and could not respond to the question related to the satisfaction. Open-ended questions about the reasons for satisfaction or dissatisfaction were asked, and several detailed responses about the inconveniences and advantages of their current CTMS were collected (Q7). The inconveniences noted by most respondents included functional limits, an interface that was not userfriendly, time-consuming problems caused by loading delays and slow run times, an unstable system that caused a number of errors, the need for additional work, a lack of integration, and low cost-effectiveness. In contrast, the advantages that were highly remarked upon by respondents were its ease of use and the simple management of clinical trial data, its usefulness for the oversight and management of projects, its functionality that enables sharing of data and schedules, the re-duced time investment compared with paper-based systems, and the partial linkage to the electronic medical record (EMR) data.
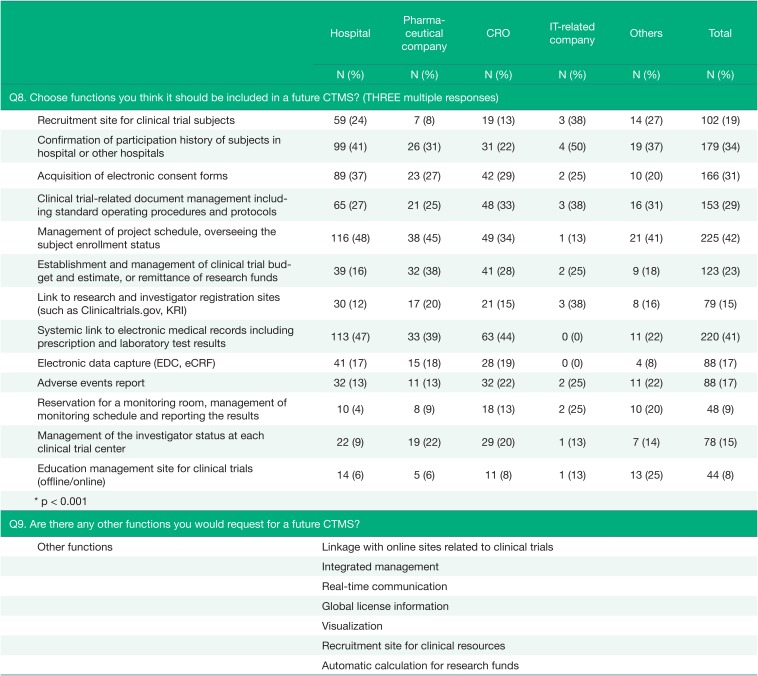
A question regarding the functions that the respondents thought should be included in a future CTMS was asked of all respondents (Q8), and the results were presented in Table 3. The most requested function was one related to management of the project schedule and management of subject enrollment status (42%), which is generally needed in clinical trials. The percentage of answers requesting systemic linkage to EMRs, including prescription and laboratory test results, was particularly large in hospitals and CROs (47% and 44%). For the respondents from hospitals, a feature related to managing subjects was also requested in a large portion (48%).
There were additional opinions about functions in the Q9, such as linkage with other systems, especially with institutional review board (IRB) systems and other related online sites; requests were also made for integrated functions that could monitor and manage clinical trials, a function for tracking medical records and drug administration history, and a function to send short message service (SMS) items directly in the system.
This survey showed that CTMSs were used mostly in CROs and pharmaceutical companies. The main barrier to the use of a CTMS was its high cost and, seemingly, lack of necessity. Approximately two-thirds of CTMS users expressed satisfaction with their current CTMS, in spite of the inconveniences and high cost. The most requested feature was that of managing the project schedule and subject enrollment status.
The result regarding the percentage of CTMS usage was similar to that of a study concerning the use of electronic data capture (EDC) in clinical trial-related institutions in Korea.[8] In the initial introduction step for EDC, the proportion of users from CROs was the highest, followed by the proportion of users from pharmaceutical companies. As the pharmaceutical market has become globalized, multi-national and multi-center clinical trials have increased, and multinational CROs have become active in Korea.[3] These CROs already have a system for monitoring clinical trial projects and sharing data with stakeholders. However, the rules and regulations regarding the management of medical data are quite strict; as a result, hospitals adopted a conservative attitude and were delayed in adopting relatively new systems, such as EDCs or CTMSs.
However, the users in hospitals used more diverse functions than the users in pharmaceutical companies and CROs, though there was no difference in the users' satisfaction with their current CTMS between workplaces. In this respect, it is expected that if investigators in hospitals use a CTMS with adequate functions, many clinical trial-related tasks are likely to be managed well by the CTMS. Since the main reason for not using a CTMS in hospitals was because of its high cost, it can be assumed that the majority of respondents will consider using a CTMS if its price is reasonable.
According to the results for the optional question regarding the users' satisfaction with their current CTMS, one can assume that the respondents used a diverse spectrum of CTMSs with different levels of quality. For example, the answers regarding the users' experiences were both positive and negative (e.g., the CTMS is not user-friendly vs. the CTMS is easy to use). As the objective of developing an integrated CTMS is to improve the overall environments of clinical trial institutes, the gap in the performance and quality of CTMSs can be decreased using new integrated CTMSs. However, the most frequently cited problems were those involving the instability of the system itself and its incompatibility with other systems.
For the functions that should be included in a future CTMS, respondents mostly answered regarding functions to manage project schedules and enrollment of subjects, to link with EMR data, and to confirm the participation history of subjects. In addition, they requested linkage with electronic IRBs (e-IRBs). Because many parts of clinical trials are related to medical care, the linkage between clinical trial data and medical data seems to be important.[91011] In Korea, the linkage between the two systems has been almost impossible due to the related regulations that emphasize the privacy of the patients over all else, but there has been a positive change. In February 2016, the Korean Ministry Of Health and Welfare revised the Enforcement Rule of the Medical Service Act, allowing medical institutions to utilize cloud computing systems to store and manage electronic medical records in an external location.[12] Although the EMR system and its equipment that store back-up data have to be in a domestic location and consent must be obtained from each patient for the use of those clinical data, this change can be regarded as a great movement toward smart data management and utilization.
As a subsequent study of this research and the aspect of the smart clinical trial core platform, the development of an innovative CTMS (iCTMS) based on integrated information in the institutes is underway. This project is funded by the Korea Clinical trials Global Initiative (KCGI) and is conducted by Seoul National University Hospital. The iCTMS will contain the main features of CTMSs, such as project management, including relative information, management of subjects and clinical trial resources, and linkage with e-IRB systems.[13] Using these programs and platforms, institutions will be able to conduct clinical trials more effectively, and high-quality clinical trial data will be stored and used.
An integrated CTMS must verify its internal stability and interoperability with other systems and must be priced reasonably so that the system can be adapted into institutes at various scales. The utilization of the CTMS will improve the environment of overall institutes conducting clinical trials. With the integrated CTMS and high-quality clinical trial data, it is expected that global competitiveness for the clinical trials in Korea will be enhanced once again.
References
1. Integrated addendum to ICH E6 (R1): Guideline for Good Clinical Practice E6 (R2). Accessed 2 February 2018. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf/.
2. Status of clinical trials overseas. Accessed 25 May 2018. https://www.koreaclinicaltrials.org/kr/contents/datainfo_data_01_tab03/view.do/.
3. START WITH KOREA. Accessed 21 May 2018. https://www.konect.or.kr/upload/downfile/Start_with_Korea_2017.pdf/.
4. Background and Necessity. Accessed 23 May 2018. http://eng.kcgi.or.kr/sub1/index2.htm/.
5. Announcement of Clinical Trial Strengthening Plan of global competitiveness. Accessed 6 February 2018. http://www.mohw.go.kr/react/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&CONT_SEQ=325129&page=1/.
6. THE POWER OF CTMS. Accessed 20 February 2018. http://www.oracle.com/us/industries/life-sciences/oracle-life-sciences-ctms-1625650.pdf.
7. Park YR, Yoon YJ, Koo H, Yoo S, Choi CM, Beck SH, et al. Utilization of a Clinical Trial Management System for the Whole Clinical Trial Process as an Integrated Database: System Development. J Med Internet Res. 2018; 20:e103. DOI: 10.2196/jmir.9312. PMID: 29691212.

8. Wang BR, Choi IY. Current State and Applications of the Electronic Clinical Trial Process in Korea. J Korea Contents Assoc. 2013; 13:281–289.

9. Effoe VS, Katula JA, Kirk JK, Pedley CF, Bollhalter LY, Brown WM, et al. The use of electronic medical records for recruitment in clinical trials: findings from the Lifestyle Intervention for Treatment of Diabetes trial. Trials. 2016; 17:496. PMID: 27733193.

10. Weng C, Li Y, Berhe S, Boland MR, Gao J, Hruby GW, et al. An Integrated Model for Patient Care and Clinical Trials (IMPACT) to support clinical research visit scheduling workflow for future learning health systems. J Biomed Inform. 2013; 46:642–652. PMID: 23684593.

11. Campion TR Jr, Blau VL, Brown SW, Izcovich D, Cole CL. Implementing a Clinical Research Management System: One Institution's Successful Approach Following Previous Failures. AMIA Jt Summits Transl Sci Proc. 2014; 2014:12–17. PMID: 25954570.
12. Choi YJ. A Study on the Regulation and Legal Issues of Medical Information Cloud Computing : Comparison with Proposal for General Data Protection Regulation of 2012 in the European Union. Eur Const. 2017; 25:105–138.
13. KCGI starts to develop the smart clinical trial core platform. Accessed 16 May 2018. http://www.kcgi.or.kr/sub4/data/read.htm?bn=data&fmlid=225&pkid=15&board_no=225&thisPage=1&startTextId=&buffer=/.
Figure 1
Current utilization of CTMSs by workplace. The total number of responses was 531. Each percentage of stacked bars was calculated for the number of respondents of each workplace.
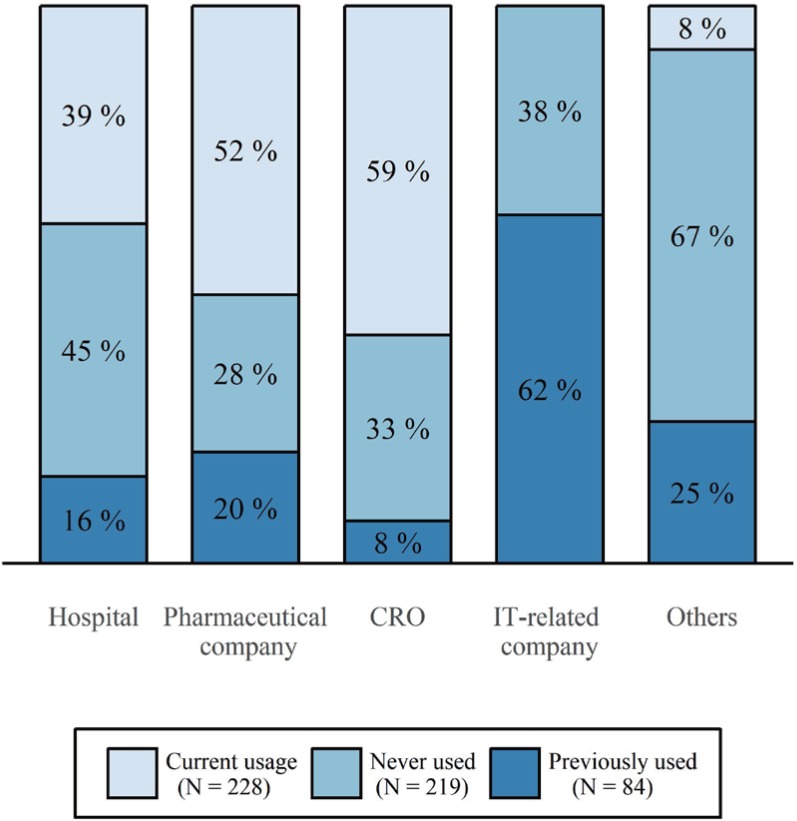
Figure 2
The difference between the reasons for not using CTMSs among respondents who were not currently using a CTMS according to past usage. The question addressed the respondents' reasons for currently not using a CTMS, and multiple responses were allowed. Because the percentages were calculated by the number of answers divided by the number of respondents, the sum of percentages is over 100.
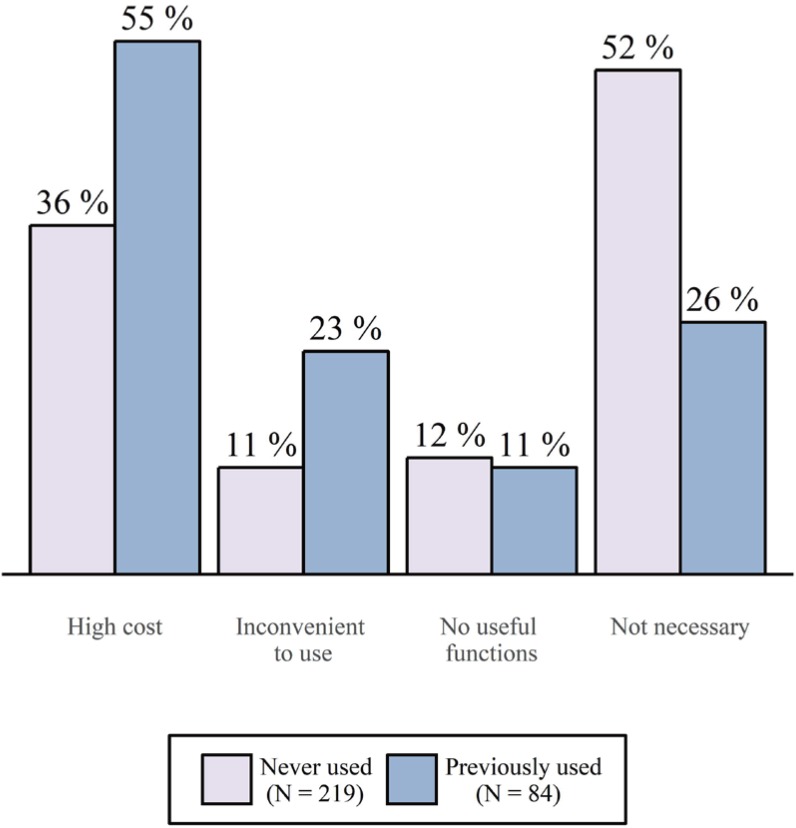
Table 1
The characteristics of respondents (Q1~Q2)
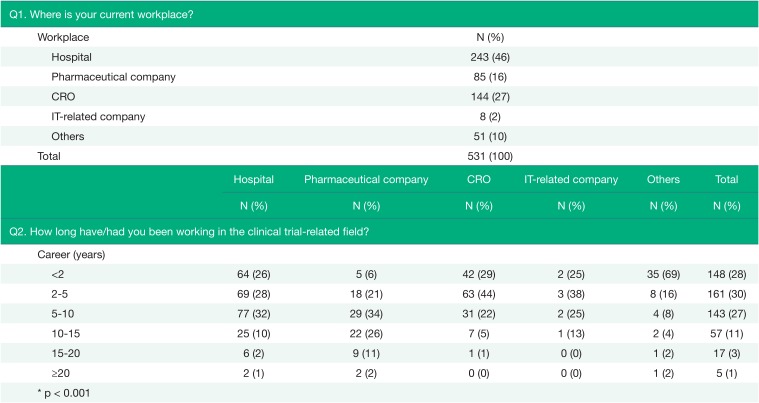
| Q1. Where is your current workplace? | ||||||
|---|---|---|---|---|---|---|
| Workplace | N (%) | |||||
| Hospital | 243 (46) | |||||
| Pharmaceutical company | 85 (16) | |||||
| CRO | 144 (27) | |||||
| IT-related company | 8 (2) | |||||
| Others | 51 (10) | |||||
| Total | 531 (100) | |||||
Table 2
The status of usage of the CTMS (Q3~Q7)
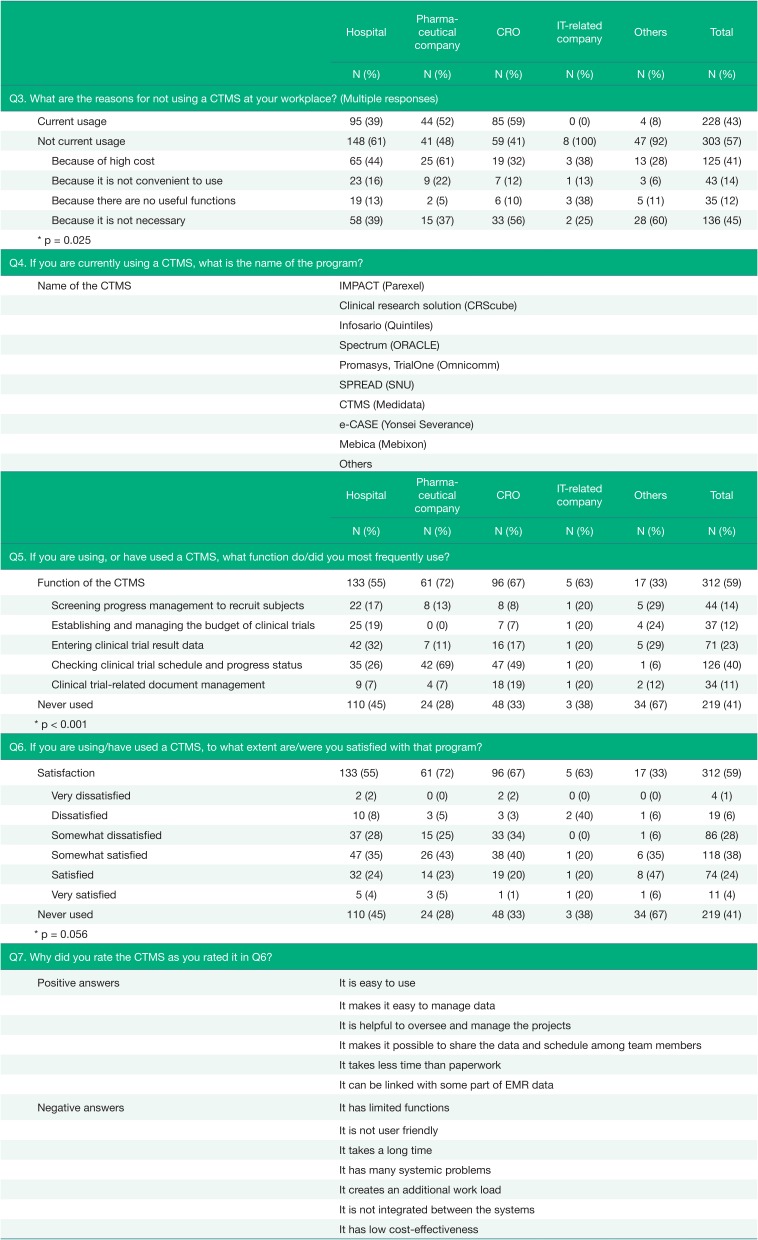
Table 3
The requirements for the future CTMS (Q8~Q9)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download