Abstract
Acknowledgements
Notes
Conflict of Interest: -Authors: BackHwan Lee is an employee of Alvogen Korea Co., Ltd., Republic of Korea. Other authors have disclosed that they have no significant relationship with or financial interests in any commercial companies related to this study.
-Reviewers: Nothing to declare
-Editors: Nothing to declare
References
Figure 1
Patient disposition from screening to completion or withdrawal in the fixed-dose combination tablet of rosuvastatin 20 mg / ezetimibe 10 mg or coadministration of rosuvastatin 20 mg and ezetimibe 10 mg.
Figure 2
Mean ± SD plasma concentration-time profiles of rosuvastatin after a single oral administration of fixed dose combination tablet (rosuvastatin 20 mg / ezetimibe 10 mg) or coadministration of rosuvastatin 20 mg and ezetimibe 10 mg. FDC = fixed dose combination. (A) linear scale (B) semi-log scale.
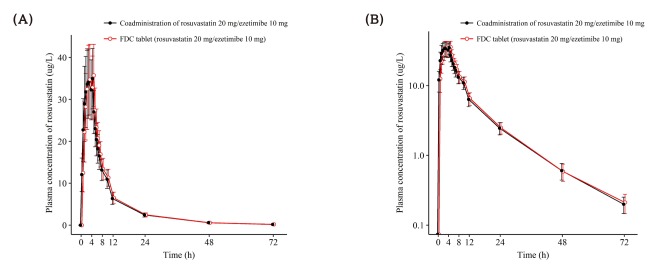
Figure 3
Mean ± SD plasma concentration-time profiles of ezetimibe after a single oral administration of fixed dose combination tablet (rosuvastatin 20 mg / ezetimibe 10 mg) or coadministration of rosuvastatin 20 mg and ezetimibe 10 mg. FDC = fixed dose combination. (A) linear scale (B) semi-log scale.
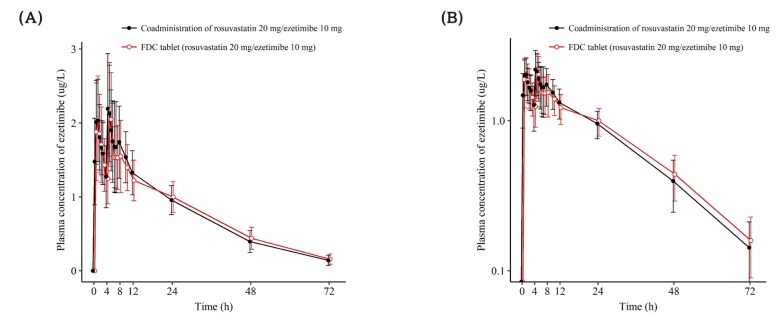
Figure 4
Mean ± SD plasma concentration-time profiles of total ezetimibe after a single oral administration of fixed dose combination tablet (rosuvastatin 20 mg / ezetimibe 10 mg) or coadministration of rosuvastatin 20 mg and ezetimibe 10 mg. FDC = fixed dose combination. (A) linear scale (B) semi-log scale.
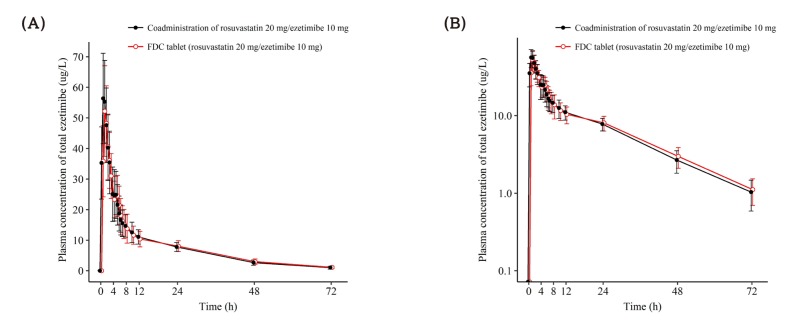
Figure 5
Individual concentration-time profile of rosuvastatin after a single oral administration of (A) fixed dose combination tablet (rosuvastatin 20 mg / ezetimibe 10 mg) and (B) coadministration of rosuvastatin 20 mg and ezetimibe 10 mg.
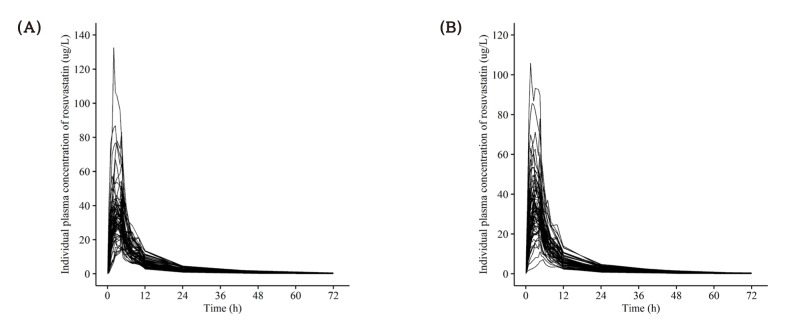
Figure 6
Individual concentration-time profile of ezetimibe after a single oral administration of (A) fixed dose combination tablet (rosuvastatin 20 mg / ezetimibe 10 mg) and (B) coadministration of rosuvastatin 20 mg and ezetimibe 10 mg.
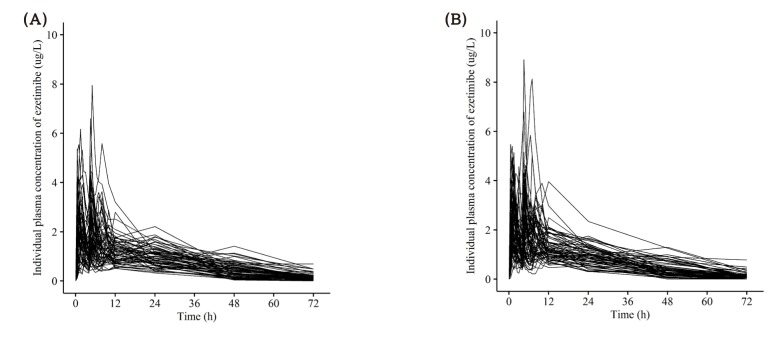
Figure 7
Individual (A) Cmax and (B) AUClast of rosuvastatin, (C) Cmax and (D) AUClast of ezetimibe, (E) Cmax and (F) AUClast of total ezetimibe after a single oral administration of fixed dose combination tablet (rosuvastatin 20 mg / ezetimibe 10 mg) or coadministration of rosuvastatin 20 mg and ezetimibe 10 mg. FDC = fixed dose combination.
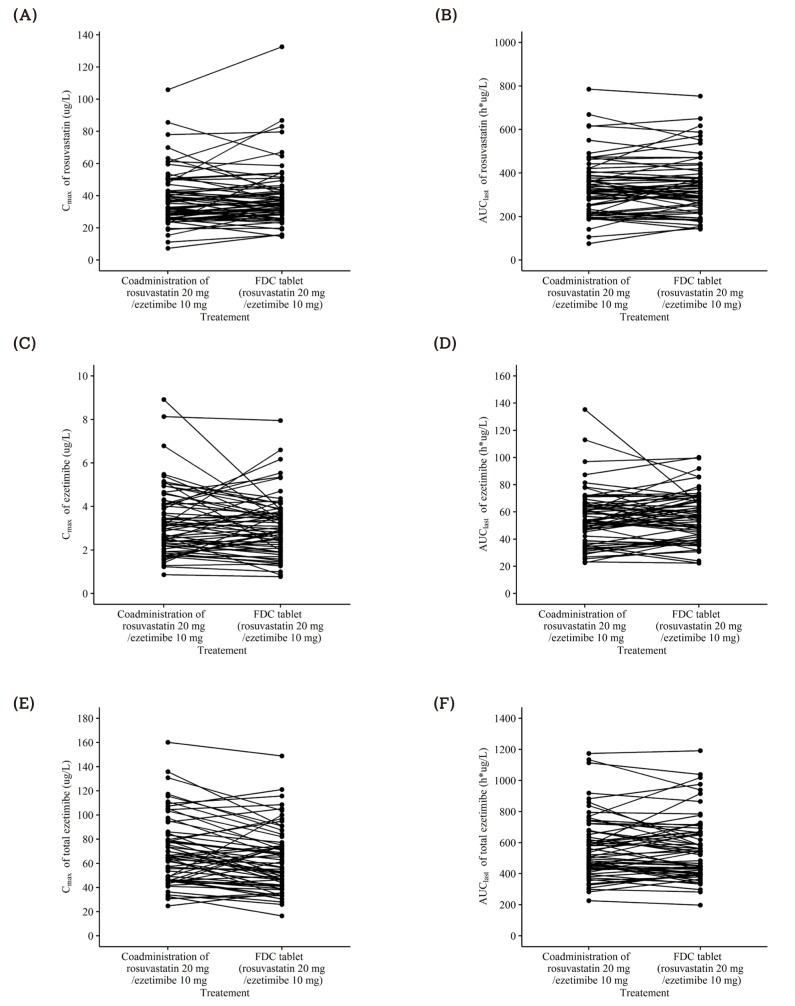
Table 1
Summary of pharmacokinetic parameters of rosuvastatin after a single oral administration of fixed-dose combination tablet (rosuvastatin 20 mg / ezetimibe 10 mg) or individual tablets (rosuvastatin 20 mg + ezetimibe 10 mg)
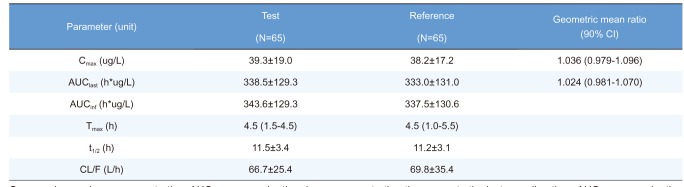
Cmax, maximum plasma concentration; AUClast, area under the plasma concentration-time curve to the last sampling time; AUCinf, area under the plasma concentration time-curve to infinity; tmax, time to Cmax; t1/2, elimination half-life; CL/F = apparent clearance.
Pharmacokinetic parameters are expressed as mean ± SD except for Tmax, which is expressed as median (range).
Table 2
Summary of pharmacokinetic parameters of ezetimibe after a single oral administration of fixed-dose combination tablet (rosuvastatin 20 mg / ezetimibe 10 mg) or individual tablets (rosuvastatin 20 mg + ezetimibe 10 mg)
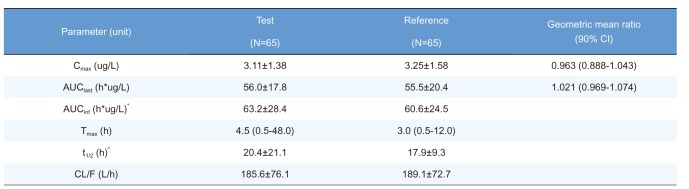
Cmax, maximum plasma concentration; AUClast, area under the plasma concentration-time curve to the last sampling time; AUCinf, area under the plasma concentration time-curve to infinity; tmax, time to Cmax; t1/2, elimination half-life; CL/F, apparent clearance.
Pharmacokinetic parameters are expressed as mean ± SD except for Tmax, which is expressed as median (range).
*n=63, the elimination rate constant of two subjects cannot be calculated.
Table 3
Summary of pharmacokinetic parameters of total ezetimibe after a single oral administration of fixed-dose combination tablet (rosuvastatin 20 mg / ezetimibe 10 mg) or individual tablets (rosuvastatin 20 mg + ezetimibe 10 mg)
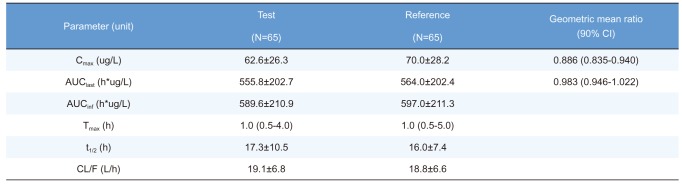
Cmax, maximum plasma concentration; AUClast, area under the plasma concentration-time curve to the last sampling time; AUCinf, area under the plasma concentration time-curve to infinity; tmax, time to Cmax; t1/2, elimination half-life; CL/F, apparent clearance.
Pharmacokinetic parameters are expressed as mean ± SD except for Tmax, which is expressed as median (range).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download