Abstract
UI14SDF100CW is a chewable tablet of sildenafil citrate, which was developed to improve compliance through convenience of administration. The purpose of this study was to compare the pharmacokinetic (PK) properties of sildenafil citrate chewable tablets (UI14SDF100CW) and conventional sildenafil citrate film-coated tablets (Viagra®, Pfizer). A randomized, open-label, single dose, two-treatment, two-period, two-way crossover study was conducted in 60 healthy male volunteers. In each period, the subjects received a single oral dose of UI14SDF100CW or Viagra® (both tablets contain 140.45 mg of sildenafil citrate, which is equivalent to 100 mg of sildenafil). Serial blood samples were collected up to 24 h post-dose for PK analysis. The plasma concentration of sildenafil was determined using a validated HPLC-MS/MS assay. PK parameters of sildenafil were calculated using non-compartmental methods. The plasma concentration-time profiles of sildenafil in both formulations were similar. For UI14SDF100CW, the Cmax and AUClast of sildenafil were 1068.69 ± 458.25 (mean ± standard deviation) mg/L and 3580.59 ± 1680.29 h·mg/L, and the corresponding values for Viagra® were 1146.84 ± 501.70 mg/L and 3406.35 ± 1452.31 h·/L, respectively. The geometric mean ratios (90% confidence intervals) of UI14SDF100CW to Viagra® for Cmax and AUClast were 0.933 (0.853–1.021) and 1.034 (0.969–1.108), respectively, which met the bioequivalence criteria of Korean regulatory agency. In conclusion, UI14SDF100CW and Viagra® showed similar PK properties. Therefore, UI14SDF100CW can be an alternative to sildenafil for the treatment of erectile dysfunction, providing better compliance.
Erectile dysfunction (ED) is defined as the inability to achieve or maintain an erection sufficient for satisfactory sexual performance. The prevalence of ED varies between countries or studies, and the reported prevalence of ED was 81.1% in Japan, 69.8% in Italy, 39.9% in Brazil, and 84.3% in Republic of Korea. [12]
Nitric oxide/cyclic guanosine monophosphate (NO-cGMP) mechanism plays an important role in mediating corporal smooth muscle relaxation required for erectile function during sexual stimulation. Sildenafil (Viagra®, Pfizer), a film-coated tablet, is a selective inhibitor of cGMP-specific phosphodiesterase type 5 (PDE-5), and is widely used for the treatment of ED. It enhances the natural NO-cGMP mechanism of penile erection following sexual stimulation.[34] Sildenafil is rapidly absorbed after oral administration, and the time taken to reach the maximal concentration (tmax) is within approximately 1 h. Its half-life is approximately 4 h.[5] It is predominately eliminated by hepatic metabolism and cleared predominantly by cytochrome P 450 (CYP) 3A4 (major route) and CYP2C9 (minor route).[46]
Conventional tablet formulations, including film-coated tablet, is convenient, economic, and stable, but may lead to poor patient compliance owing to swallowing difficulties, especially in geriatric patients. Therefore, there is an unmet medical need for a more comfortable formulation.
For enhanced compliance, UI14SDF100CW, a chewable sildenafil tablet, was developed to improve compliance through convenience of administration. The aim of this study is to compare the pharmacokinetic (PK) properties of sildenafil citrate chewable tablet (UI14SDF100CW) and sildenafil citrate film-coated tablet (Viagra®).
A randomized, open-label, single dose, two-treatment, two-period, two-sequence crossover study was conducted in 60 healthy Korean male volunteers. The study protocol was registered at CRIS, the registry run by Korean government (https://cris.nih.go.kr No. KCT0002340) after approval by the Institutional Review Board of Chungnam National University Hospital (No. CNUH 2012-01-021). All subjects provided written informed consent before any procedure, and the study was conducted in accordance with the major ethical principles stipulated in the Declaration of Helsinki and Good Clinical Practice guidelines.
The subjects who met the following criteria were included in this study: age between 20 and 55 years; weight at least 55 kg and within 20% of ideal body weight; and no clinically significant abnormalities based on medical history, physical examination, and clinical laboratory tests. Sixty subjects were randomized to two treatment sequences, in which the treatments consisted of a single oral dose of the test drug (UI14SDF100CW) or the reference drug (sildenafil citrate film-coated tablet, Viagra®). Both tablets contain 140.45 mg of sildenafil citrate, which is equivalent to 100 mg sildenafil.
The subjects were admitted to the Chungnam National University Hospital Clinical Trial Center for 3 days in each period with a 10-day washout period. After overnight fasting, the test drug was administered with 50 mL of water or the reference drug was administered with 240 mL of water. Food was restricted from 10 h pre-dose until 4 h post-dose, and water was not permitted until 2 h following dosing.
To determine the plasma concentration of sildenafil, blood samples were collected up to 24 h after dosing in each period: at pre-dose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 h post-dose. The collected blood samples were centrifuged at 3,000 rpm and 4℃ for 10 min, and the obtained plasma samples were frozen below -70℃ until assay.
Plasma concentrations of sildenafil were determined using high-performance liquid chromatography (Agilent 1200 series; Agilent Technologies, Waldbronn, Germany) system, coupled with a tandem mass spectrometry method (Agilent 6410A; Waldbronn, Germany) validated by Lewis et al.[7] Chromatographic separation was performed under gradient conditions using a ZORBAX SB-Ag column (2.1×100 mm, 3.5 µm; Agilent, USA). The calibration curve for sildenafil was linear over the range of 10-2,000 µg/L (r2 ≥ 0.99).The accuracy range for silde-nafil was 93.5–104.4%. The precision coefficients of variation for were ≤ 7.8%.
The following PK parameters were obtained by noncompartmental methods using Phoenix WinNonlin software version 7.0 (Certara, St Louis, MO, USA). The maximum concentration (Cmax) and tmax were obtained directly from the observed values; the area under the concentration-time curve (AUC) was determined using the linear trapezoidal method for ascending concentrations and the log trapezoidal method for descending concentrations. AUClast is the AUC from the time of last dosing to the last measureable concentration, and AUCinf is the AUC from the time of last dosing extrapolated to infinity. The terminal elimination half-life (t½) was calculated as the natural logarithm of 2 divided by the terminal elimination rate constant.
The sample size of this study (n=52) was sufficient to ensure a power of 80%, with a significance level of 5% for correctly concluding bioequivalence under the assumptions that the intra-subject coefficient of variation (CV) of sildenafil was 40.0%. Therefore, a sample size of 60 was chosen to account for possible dropouts.
To assess the regulatory requirement for bioequivalence, the Cmax and AUClast of sildenafil were log-transformed and the geometric mean ratio (GMR) and 90% confidence interval (90% CI) of the test drug to the reference drug were calculated from the analyses of variance model (ANOVA), including sequence, period, and treatment as fixed effect and subject within sequence as random effect. If the 90% CI fell in the conventional bioequivalence range of 0.800–1.250, it was concluded that there was no significant difference between the test drug and reference drug.
Tolerability and safety were evaluated throughout the study based on physical examinations, vital signs, 12-lead electrocardiograms, and clinical laboratory tests, including hematology, serum chemistry, and urinalysis. Adverse events (AEs) were collected throughout the study and assessed to determine their relationship to the treatment by the investigators.
Three of the 60 subjects withdrew consent owing to personal reasons after the first dosing, and 57 subjects completed the study. Therefore, 60 subjects were enrolled for safety analysis, and 57 subjects were enrolled for PK analysis. The age, height, and weight of the 60 enrolled subjects were 24.6 ± 2.1 (mean ± standard deviation) years, 177.1 ± 5.6 cm, and 68.0 ± 8.3 kg, respectively (Table 1).
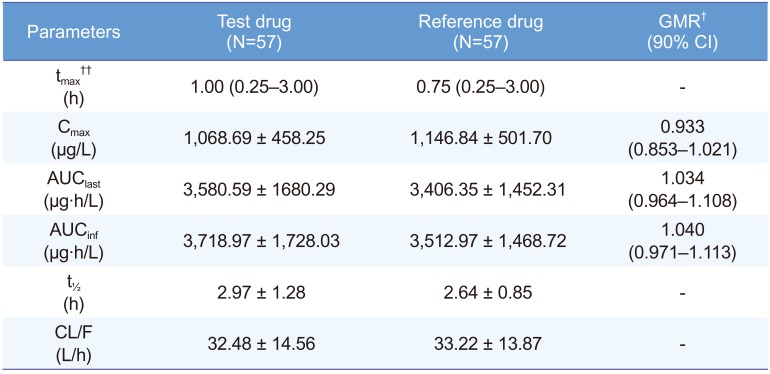
The sildenafil plasma concentration-time curves of the test and reference drugs were similar (Fig. 1) The PK parameters were also similar between the two formulations (Table 2).
In the statistical aspects of bioequivalence, the log-transformed Cmax and AUClast indicated no significant differences between the test drug and reference drug. The GMR (90% CI) of the test drug to the reference drug for Cmax and AUClast were 0.933 (0.853–1.021) and 1.034 (0.964– 1.108), respectively, which are within the Korean bioequivalence criterion of 0.80 to 1.25 (Table 2).
Twenty-five AEs (abnormal vision, headache, dizziness, hot flashes, nausea, and diarrhea) occurred after the administration of the test drug in 17 subjects, whereas 29 AEs (abnormal vision, headache, dizziness, and hot flashes) occurred after the administration of the reference drug in 21 subjects. All the AEs were mild in intensity and resolved spontaneously without any intervention. There was no statistical difference in AEs between the test drug and reference drug.
No clinically significant changes were observed in clinical laboratory test results when compared with the baseline values. Furthermore, there were no clinically significant changes in physical examination, vital signs, and 12-lead electrocardiograms.
In this study, the CV values for Cmax and AUClast were 42.8–43.7% and 42.6–46.9%, respectively, which were similar to those reported in previous PK studies of sildenafil.[89] In addition, intra-subject CV for Cmax and AUClast were 22.4 and 29.7%, respectively, which were assessed by repeated measures analysis and conducted using the SAS Proc Mixed Function of Generalized Linear Models. Based on the results of this study, a sample size of more than 30 will be sufficient to ensure a power of over 80% for correctly concluding the bioequivalence study.
In a few previous PK studies, CV of sildenafil was reported to be above 60.0%, and therefore might be considered a highly variable drug.[1011] Considering the regulatory guideline of a highly variable drug, the bioequivalent study of highly variable drugs can be designed as a fully or partial replicate design. In a fully replicate design or partial replicate design of sildenafil bioequivalent study, a sample size of 16 or 24 may be sufficient to ensure a power of 80% for correctly concluding bioequivalence under the following assumption: a significance level of 5% and a CV of 30.0%.
In conclusion, UI14SDF100CW, a sildenafil citrate chewable tablet, demonstrated PK properties similar to Viagra®, a conventional sildenafil citrate film-coated tablet. Therefore, UI14SDF100CW can be an alternative option to sildenafil for the treatment of ED, offering comfortable administration.
Acknowledgements
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1063). This work was also supported by a research fund of Chungnam National University and supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B04033515).
References
1. Yang DH, Jeong JY, Jang SN, Lee SK, Choi YJ, Kim DH. Prevalence and Risk Factors for Erectile Dysfunction in Aging Men: Hallym Aging Study (HAS). Korean J Urol. 2007; 48:1258–1276.

2. Nehra A, Kulaksizoglu H. Global Perspectives and Controversies in the Epidemiology of Male Erectile Dysfunction. Curr Opin Urol. 2002; 12:493–496. PMID: 12409879.

3. Boolell M, Allesn MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, et al. Sildenafil: an orally active type 5 cyclic GMP177 specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996; 8:47–52. PMID: 8858389.
4. Moreland RB, Goldstein I, Traish A. Sildenafil, a novel inhibitor of phosphodiesterase type 5 in human corpus cavernosum smooth muscle cells. Life Sci. 1998; 62:PL 309–PL 318.

5. Eardley I, Ellis P, Boolell M, Wulff M. Onset and duration of action of sildenafil citrate for the treatment of erectile dysfunction. Br J Clin Pharmacol. 2002; 53(Suppl 1):61S–65S. PMID: 11879261.
6. Gupta M, Kovar A, Meibohm B. The Clinical Pharmacokinetics 186 of Phosphodiesterase-5 Inhibitorsfor Erectile Dysfunction. J Clin Pharmacol. 2005; 45:987–1003. PMID: 16100293.
7. Lewis JR, Johnson DR. A novel method for the Determination of sildenafil (Viagra) and its metabolite (UK-103, 320) in postmortem specimen using LC-MS/MS and LC-MS/MS.MS, A final reprt by U.S Department fo Transport Federal Aviation administration. 2000. 5. Accessed Aug 10 2017. https://www.faa.gov/data_research/med_humanfacs/oamtechreports/2000s/media/00_20.pdf.
8. Kanjanawart S, Kongyingyoes B, Gaysornsiri D, Tangsucharit P, Puapairoj P, Vannaprasaht S, et al. Bioequivalence and Pharmacokinetic Study of Sildenafil in Healthy Thai Male Volunteers. Srinagarind Med J. 2008; 23:38–44.
9. Valenzuela F, Davila G, Ibañez Y, Garcia L, Crownover P, Gómez-Palacio R, et al. Relative Bioavailability of Chewable and Conventional Film-Coated Tablet Formulations of Sildenafil 100mg in Healthy Volunteers Under Fasting Conditions. J Bioequiv Availab. 2011; 3:207–210.
10. Stavros F, Kramer WG, Wilkins MR. The effects of sitaxentan on sildenafil pharmacokinetics and pharmacodynamics in healthy subjects. Br J Clin Pharmacol. 2010; 69:23–26. DOI: 10.1111/j.1365-2125.2009.03541.x. PMID: 20078609.

11. European Medicines Agency, ASSESSMENT REPORT FOR Sildenafil ratiopharm. 2009. Accessed Aug 10 2017. http://www.ema.europa.eu/docs/en_GB/document_library/PAR_-_Public_assessment_report/human/001080/WC500068025.pdf.
Figure 1
Mean plasma concentration-time curves of sildenafil [(a) linear scale, (b) log scale] after a single oral dose of the test drug (UI14SDF100CW) or the reference drug (Viagra®). Error bars represent standard deviations.
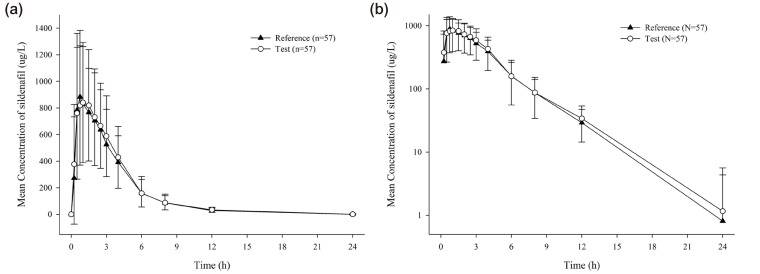
Table 1
Demographics of the enrolled subjects
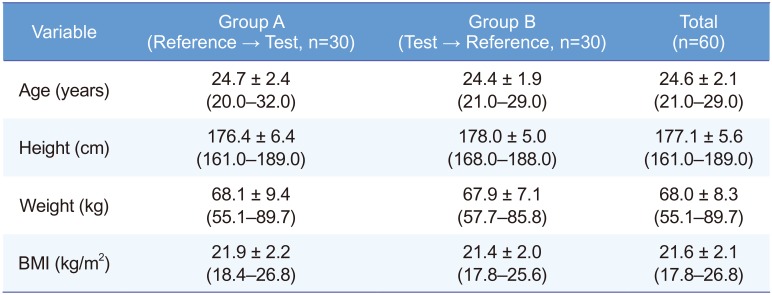
Table 2
Pharmacokinetic parameters of sildenafil after a single oral dose of the test drug (UI14SDF100CW) or the reference drug (Viagra®)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download