Abstract
Cilostazol controlled-release (CR) tablets have recently been developed by Korea United Pharm (Seoul, Korea). The tablets use a patented double CR system, which improves drug compliance by allowing “once daily” administration and reduces adverse events by sustaining a more even plasma concentration for 24 h. We conducted an open, randomized, two-period, two-treatment, crossover study to compare the pharmacokinetic (PK) characteristics and tolerability of cilostazol when administered to healthy Korean male volunteers as CR or immediate release (IR) tablets (Pletal, Korea Otsuka Pharmaceutical Co., Gyeonggi-do, Korea). Each volunteer was randomly allocated to receive a single tablet of cilostazol CR (200 mg) or two tablets of cilostazol IR (100 mg) with a 7-day washout period between treatments. Plasma cilostazol, OPC-13015 (3,4-dehydrocilostazol), and OPC-13213 (4'-trans-hydroxycilostazol) were assayed using liquid chromatography-tandem mass spectrometry for PK analysis. Thirty participants completed the study with no clinically relevant safety issues. The peak concentrations (Cmax, mean ± SD) of cilostazol CR and cilostazol IR were 1414.6 ± 49.3 and 1413.1 ± 35.2 ng/mL, respectively, and the areas under the plasma concentration–time curve from 0 to the last concentration (AUClast) were 23928.7 ± 65.9 and 25312.0 ± 62.6 ng∙h/ mL, respectively. The geometric mean ratios (cilostazol CR/ cilostazol IR, GMR) of the Cmax and AUClast values were 1.001 (90% CI: 0.822, 1.220) and 0.945 (90% CI: 0.814, 1.098), respectively. The frequencies of adverse events were similar. The present study showed that cilostazol PK and tolerability were comparable when administered to healthy Korean men, regardless of whether administered as cilostazol CR or IR.
REFERENCES
2.Horie N., Kaminogo M., Izumo T., Hayashi K., Tsujino A., Nagata I. Cilostazol may prevent cardioembolic stroke in patients undergoing antiplatelet therapy. Neurol Res. 2015. 37:619–623. DOI: doi: 10.1179/1743132815Y.0000000021.

3.Liu JS., Chuang TJ., Chen JH., Lee CH., Hsieh CH., Lin TK, et al. Cilostazol attenuates the severity of peripheral arterial occlusive disease in patients with type 2 diabetes: the role of plasma soluble receptor for advanced glycation end-products. Endocrine. 2015. 49:703–710. DOI: doi: 10.1007/s12020-015-0545-6.

4.Tanaka T., Ishikawa T., Hagiwara M., Onoda K., Itoh H., Hidaka H. Effects of cilostazol, a selective cAMP phosphodiesterase inhibitor on the contraction of vascular smooth muscle. Pharmacology. 1988. 36:313–320.

5.Dawson DL., Cutler BS., Meissner MH., Strandness DE Jr. Cilostazol has beneficial effects in treatment of intermittent claudication. Results from a multi-center, randomized, prospective, double-blind trial. Circulation. 1998. 98:678–686.

6.Dawson DL., Cutler BS., Hiatt WR., Hobson RW 2nd., Martin JD., Bortey EB, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J med Med. 2000. 109:523–530.

7.Shin KH., Yoon G., Yoon IS., Park JW. Preparation and evaluation of oral controlled-release cilostazol formulation: pharmacokinetics and antithrombotic efficacy in dogs and healthy male Korean participants. J Pharm Pharmacol. 2014. 66:961–974. DOI: doi: 10.1111/jphp.12227.

8.Karalis V., Macheras P. Examining the role of metabolites in bioequivalence assessment. J Pharm Pharm Sci. 2010. 13:198–217.

9.Srinivas NR. Considerations for metabolite pharmacokinetic data in bioavail-ability/bioequivalence assessments. Overview of the recent trends. Arzneimit-telforschung. 2009. 59:155–165. DOI: doi: 10.1055/s-0031-1296380.
10.Jackson AJ., Robbie G., Marroum P. Metabolites and bioequivalence: past and present. Clin Pharmacokinet. 2004. 43:655–672.
11.Kim HS., Kim GY., Yeo CW., Oh MK., Ghim JL., Shon JH, et al. The effect of Ginkgo biloba extracts on the pharmacokinetics and pharmacodynamics of cilostazol and its active metabolites in healthy Korean subjects. Br J Clin Pharmacol. 2014. 77:821–830. DOI: doi: 10.1111/bcp.12236.
12.Kim YH., Ghim JL., Jung JA., Cho SH., Choe S., Choi HY, et al. Pharmacokinetic comparison of sustained- and immediate-release formulations of cilostazol after multiple oral doses in fed healthy male Korean volunteers. Drug Des Devel Ther. 2015. 9:3571–3577. DOI: doi: 10.2147/DDDT.S86845.
13.Woo SK., Kang WK., Kwon KI. Pharmacokinetic and pharmacodynamic modeling of the antiplatelet and cardiov ascular effects of cilostazol in healthy humans. Clin Pharmacol Ther. 2002. 71:246–252.
Figure 2.
Mean plasma concentration–time profiles of cilostazol (A), OPC13015(3,4-dehydrocilostazol) (B) and OPC13213 (4´-trans-hydroxycilostazol) (C) after oral administration of a single tablet of cilostazol CR 200 mg or two tablets of cilostazol IR 100 mg in healthy Korean male study participants (n = 30). The error bars represent standard deviation. ∗Reference drug: cilostazol 100 mg IR two tablets, test drug: cilostazol 200 mg CR one tablet.
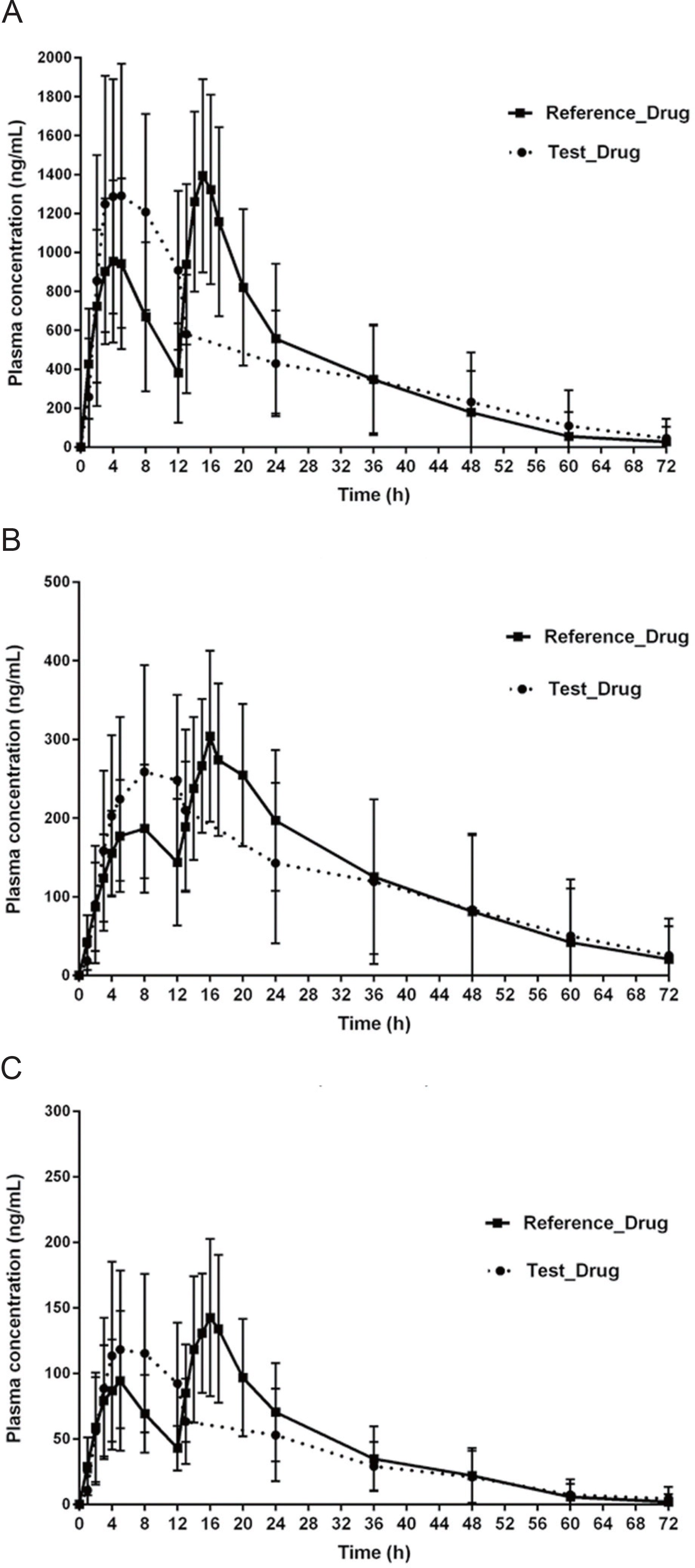
Table 1.
Demographic characteristics of the study participants
| Group A (n=15) | Group B (n=15) | Total (n = 30) | P | ||
|---|---|---|---|---|---|
| Age (years) | 22.9 ± 2.0 | 23.9 ±2.1 | 23.4 ± 2 | 0.188∗ | |
| Weight (kg) | 68.7 ± 7.1 | 66.9 ± 6.9 | 67.7 ± 6.8 | 0.471∗ | |
| Height (cm) | 176.5 ± 5.3 | 174.0 ± 4.5 | 175.2 ± 4.9 | 0.183∗ | |
| Drinking‡ | Yes | 8 | 8 | 16 | > 0.999§ |
| No | 7 | 7 | 14 | ||
| Smoking‡ | Yes | 7 | 8 | 15 | > 0.999§ |
| No | 8 | 7 | 15 | ||
| Caffeine‡ | Yes | 4 | 9 | 13 | 0.139§ |
| No | 11 | 6 | 17 |
Table 2.
Summary of pharmacokinetic parameters after a single oral administration of the two formulations
| Parameters | Cilostazol CR (n = 30) | Cilostazol IR (n = 30) | P |
|---|---|---|---|
| Cilostazol | |||
| Cmax (ng/ml) | 1414.6 (49.3) | 1413.1 (35.2) | 0.993 |
| [335.9 to 3097.7] | [420.4 to 2557.4] | ||
| AUClast (ng∙h/ml) | 23928.7 (65.9) | 25312.0 (62.6) | 0.528 |
| [9898.6 to 75046.5] | [6473.8 to 70296.3] | ||
| AUCinf (ng∙h/ml) | 28232.8 (69.2) | 28606.6 (60.6) | 0.886 |
| [13789.1 to 91534.3] | [10160.4 to 79973.5] | ||
| Tmax∗ (h) | 4.0 [2.0 to 8.0] | 15 [2.0 to 24.0] | - |
| t1/2 (h) | 15.7 (58.4) | 10.6 (68.5) | 0.017 |
| [6.1 to 47.6] | [3.2 to 38.1] | ||
| OPC-13015 | |||
| Cmax (ng/ml) | 261.1 (52.5) | 290.2 (33.2) | 0.370 |
| [94.9 to 736.6] | [86.9 to 497.1] | ||
| AUClast (ng∙h/mL) | 6968.8 (82.3) | 7630.3 (60.9) | 0.317 |
| [2991.7 to 27186.7] | [2695.0 to 22252.9] | ||
| AUCinf (ng∙h/mL) | 7348.6 (97.3) | 7890.6 (72.0) | 0.435 |
| [3130.4 to 31866.9] | [2846.0 to 28462.5] | ||
| Tmax∗ (h) | 6.0 [3.0 to 36.0] | 16 [15.0 to 36.0] | |
| t1/2 (h) | 10.3 (90.1) | 8.3 (64.6) | 0.052 |
| [5.2 to 45.0] | [4.7 to 23.9] | ||
| OPC-13213 | |||
| Cmax (ng/mL) | 121.3 (57.6) | 147.6 (43.2) | 0.046 |
| [30.6 to 326.4] | [76.8 to 349.6] | ||
| AUClast (ng∙h/mL) | 2412.6 (53.0) | 2964.0 (48.1) | 0.007 |
| [996.1 to 5552.8] | [1636.1 to 7767.6] | ||
| AUCinf (ng∙h/mL) | 2909.3 (52.8) | 3183.4 (46.9) | 0.260 |
| [1418.3 to 6648.8] | [1759.6 to 7832.2] | ||
| Tmax∗ (h) | 5.0 [3.0 to 8.0] | 16.0 [3.0 to 24.0] | - |
| t1/2 (h) | 15.6 (112.0) | 10.5 (4.6) | 0.012 |
| [6.2 to 92.7] | [4.7 to 22.7] |
Table 3.
Bioequivalence assessment of pharmacokinetic parameters




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download