Abstract
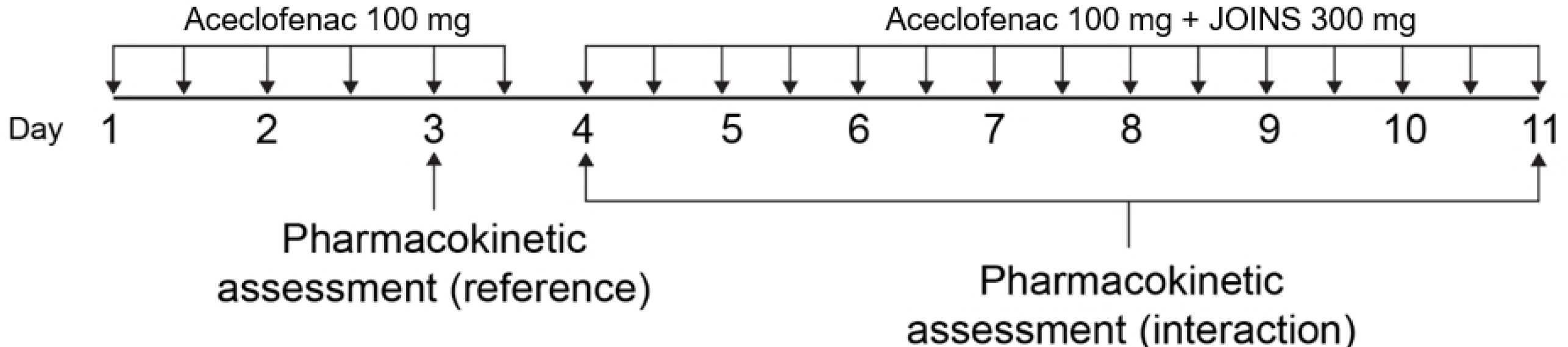
JOINS, an herbal anti-arthritic drug, was developed for the treatment and pain relief of knee osteoarthritis. It was approved for use in Korea by the Ministry of Food and Drug Safety in 2001. The aim of this study was to investigate the effect of JOINS on the pharmacokinetic (PK) profiles of aceclofenac in healthy adults. A PK drug-drug interaction study was conducted in 61 healthy subjects by using an open-label, multiple-dose, one sequence, two-period design. Blood samples were collected for plasma concentrations of aceclofenac during the reference period (aceclofenac 100 mg alone) and interaction period (aceclofenac 100 mg + JOINS 300 mg). The area under the curve within a dosing interval (τ) at steady state (AUCτ,ss) and the Cmax at steady state (Cmax,ss) of aceclofenac were analyzed by a non-compartment model using the Phoenix® WinNonlin® software version 6.3 (Pharsight, Mountain View, CA, USA). The 90% CIs of the geometric mean ratios (GMRs) of the AUCτ,ss of aceclofenac with JOINS to without JOINS (D4/D3 and D11/D3) were 0.9593–1.0130 and 0.9745–1.0291, respectively, and the corresponding values for the Cmax,ss of aceclofenac with JOINS to without JOINS (D4/D3 and D11/D3) were 0.8578–0.9795 and 0.8510–0.9717. Aceclofenac alone or co-administered with JOINS was safe and well tolerated with no serious adverse drug reactions or significant differences in the severity of adverse events (AEs) between the two treatment groups. We conclude that co-administration of aceclofenac with JOINS does not influence the PK and safety profiles of aceclofenac.
REFERENCES
2.Zhang W., Nuki G., Moskowitz RW., Abramson S., Altman RD., Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010. 18:476–499. DOI: doi: 10.1016/j.joca.2010.01.013.
3.Flood J. The role of acetaminophen in the treatment of osteoarthritis. Am J Manag Care. 2010. 16(Suppl Management):S48-S54.
4.Raza K., Kumar M., Kumar P., Malik R., Sharma G., Kaur M, et al. Topical delivery of aceclofenac: challenges and promises of novel drug delivery systems. Biomed Res Int. 2014. 2014:406731. DOI: doi: 10.1155/2014/406731.

5.Yamazaki R., Kawai S., Matsuzaki T., Kaneda N., Hashimoto S., Yokokura T, et al. Aceclofenac blocks prostaglandin E2 production following its intracellular conversion into cyclooxygenase inhibitors. Eur J Pharmacol. 1997. 329:181–187.

6.Brogden RN., Wiseman LR. Aceclofenac. A review of its pharmacodynamic properties and therapeutic potential in the treatment of rheumatic disorders and in pain management. Drugs. 1996. 52:113–124.
7.Pareek A., Chandurkar N. Comparison of gastrointestinal safety and tolerability of aceclofenac with diclofenac: a multicenter, randomized, double-blind study in patients with knee osteoarthritis. Curr Med Res Opin. 2013. 29:849–859. DOI: doi: 10.1185/03007995.2013.795139.

8.Lung YB., Seong SC., Lee MC., Shin YU., Kim DH., Kim JM, et al. .A four-week, randomized, double-blind trial of the efficacy and safety of SKI306X: a herbal anti-arthritic agent versus diclofenac in osteoarthritis of the knee. Am J Chin Med. 2004. 32:291–301.
9.Choi JH., Choi JH., Kim DY., Yoon JH., Youn HY., Yi JB, et al. .Effects of SKI 306X, a new herbal agent, on proteoglycan degradation in cartilage explant culture and collagenase-induced rabbit osteoarthritis model. Osteoarthritis Cartilage. 2002. 10:471–478.

10.Kim JH., Rhee HI., Jung IH., Ryu K., Jung K., Han CK, et al. .SKI306X, an oriental herbal mixture, suppresses gastric leukotriene B4 synthesis without causing mucosal injury and the diclofenac-induced gastric lesions. Life Sci. 2005. 77:1181–1193.

11.Rhim SY., Park JH., Park YS., Lee MH., Shaw LM., Kang JS. Bioequivalence and pharmacokinetic evaluation of two branded formulations of aceclofenac 100 mg: a single-dose, randomized, open-label, two-period crossover comparison in healthy Korean adult volunteers. Clin Ther. 2008. 30:633–640.

12.Hinz B., Auge D., Rau T., Rietbrock S., Brune K., Werner U. Simultaneous determination of aceclofenac and three of its metabolites in human plasma by high-performance liquid chromatography. Biomed Chromatogr. 2003. 17:268–275.

13.Ihm CH., Hwang IT., Kim EY., Kang WK. Pharmacokinetic Study of Aceclofenac and its Metabolites, and Application to Bioequivalence Study. Kor J Clin Pharm. 2006. 16.
Figure 2.
Mean (SD) plasma concentration-time profiles of aceclofenac with or without JOINS (upper graph: linear, lower graph: log-linear). Treatment: aceclofenac 100 mg alone (Day 3), aceclofenac 100 mg with JOINS 300 mg (Day 4 and Day 11), τ = 12 h.
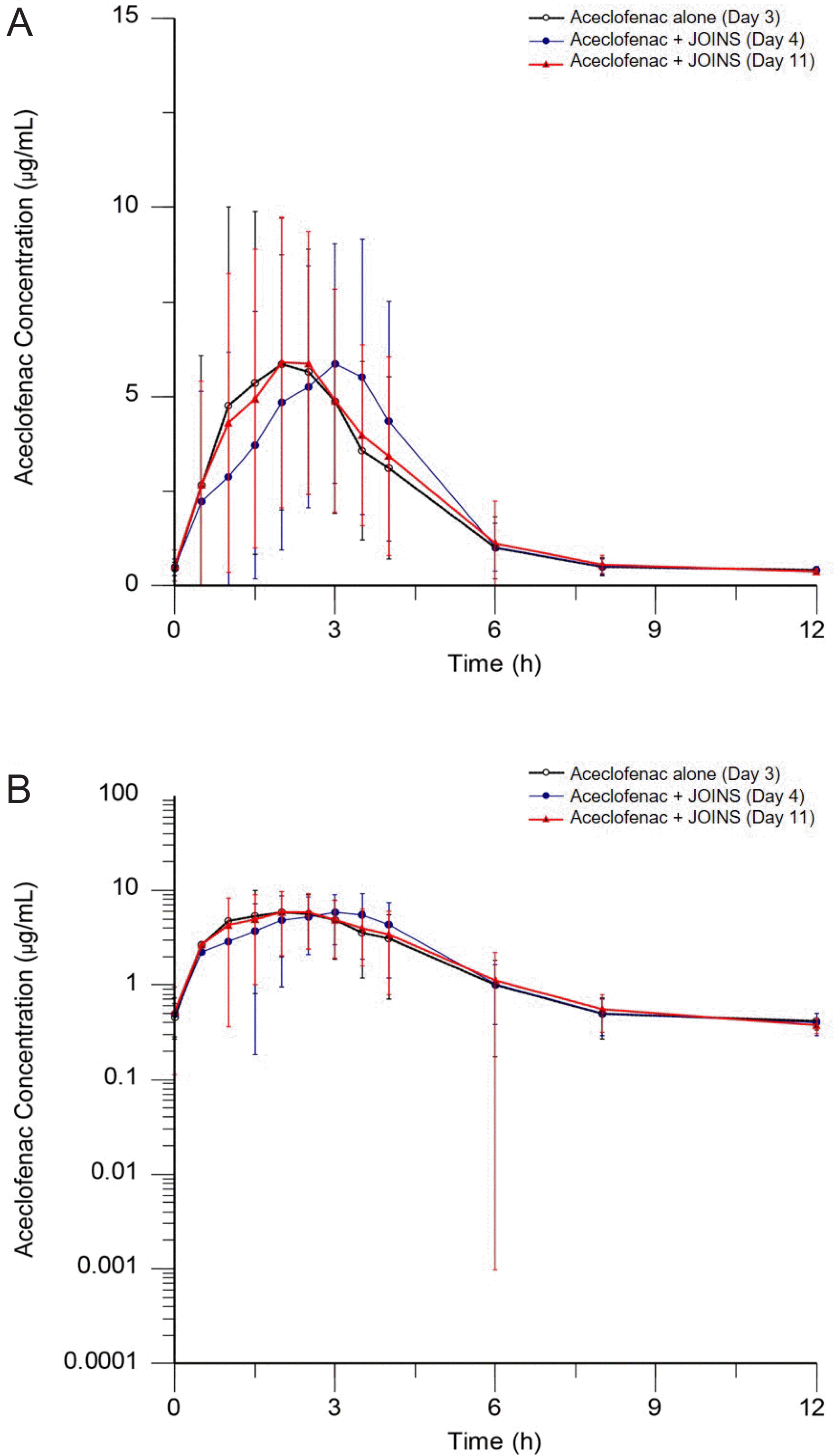
Table 1.
Characteristics of the study participants∗
Table 2.
Comparison of the pharmacokinetics of aceclofenac with and without JOINS
| Parameter |
Arithmetic mean ± SD |
Geometric mean ratio (90% CI)a |
|||
|---|---|---|---|---|---|
| Day 3 | Day 4 | Day 11 | Day 4/Day 3 | Day 11/Day 3 | |
| tmax,ss (h)∗ | 2.00 (0.50, 6.00) | 3.00 (0.50, 6.00) | 2.00 (0.50, 6.00) | - | - |
| AUCτ,ss | 22.25 ± 4.55 | 22.01 ± 4.91 | 22.34 ± 4.95 | 0.9858 | 1.0015 |
| τ (h∙μg/mL) | (0.9593–1.0130) | (0.9745–1.0291) | |||
| Cmax,ss | 10.98 ± 3.00 | 10.01 ± 2.48 | 9.90 ± 2.42 | 0.9166 | 0.9093 |
| (μg/mL) | (0.8578–0.9795) | (0.8510–0.9717) | |||
| Cmin,ss (μg/mL) | 0.18 ± 0.20 | 0.13 ± 0.19 | 0.18 ± 0.20 | - | - |
| Cavg,ss (μg/mL) | 1.91 ± 0.37 | 1.89 ± 0.40 | 1.92 ± 0.40 | - | - |
Treatment: aceclofenac 100 mg only (Day 3), aceclofenac 100 mg with JOINS 300 mg (Day 4 and Day 11), τ = 12 h.
∗ The data are presented as the median [min, max]. SD, standard deviation; CI, confidence interval; tmax, time to Cmax; AUCτ, the area under the concentration-time curve during the dosing interval; AUCinf, AUC from dosing time extrapolated to infinity; Cmax, the maximum concentration of drug; Cmin, the minimal drug concentration; Cavg, the average drug concentration during the dosing interval,




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download