Abstract
A high performance liquid chromatography (HPLC) paired with UV-vis detection method to determine ascorbic acid and its oxidation product, dehydroascorbic acid, in human plasma was developed. Ascorbic acid in human plasma was extracted and stabilized using 10% metaphosphoric acid, and was analyzed by a Symmetry C18 column with 5 mM Hexadecyltrimethylammonium bromide and 50 mM KH2PO4 solution as the mobile phase (1.0 mL/min flow rate). Isoascorbic acid served as the internal standard and ultraviolet detector wavelength was 254 nm and 265 nm. Dehydroascorbic acid concentration was calculated from the differences in ascorbic acid concentration before and after reduction by dithiothreitol reagent. Quantification for ascorbic acid in human plasma was linear from 1–100 μg/mL. The inter- and intra-day precisions and accuracy were determined and the results were found to be within ±15%. This method was successfully applied to a human pharmacokinetic study of ascorbic acid as well as dehydroascorbic acid after oral administration of 4,000 mg vitamin C tablets to healthy Korean volunteers.
References
1. Rose RC. Transport of ascorbic acid and other water-soluble vitamins. Biochim Biophys Acta. 1988; 947:335–366.

2. Torres P, Galleguillos P, Lissi E, López-Alarcón C. Antioxidant capacity of human blood plasma and human urine: simultaneous evaluation of the ORAC index and ascorbic acid concentration employing pyrogallol red as probe. Bioorg Med Chem. 2008; 16:9171–9175. doi: 10.1016/j.bmc.2008.09. 024.

4. Fox FW, Levy LF. Experiments confirming the antiscorbutic activity of dehydroascorbic acid and a study of its storage and that of ascorbic acid by the guinea-pig at different levels of intake. Biochem J. 1936; 30:211–217.

5. Wang Y, Kashiba M, Kasahara E, Tsuchiya M, Sato EF, Utsumi K, et al. Metabolic cooperation of ascorbic acid and glutathione in normal and vitamin C-deficient ODS rats. Physiol Chem Phys Med NMR. 2001; 33:29–39.
6. Lykkesfeldt J, Loft S, Nielsen JB, Poulsen HE. Ascorbic acid and dehydroascorbic acid as biomarkers of oxidative stress caused by smoking. Am J Clin Nutr. 1997; 65:959–963.

7. Lykkesfeldt J, Loft S. Poulsen HE. Determination of ascorbic acid and dehydroascorbic acid in plasma by high-performance liquid chromatography with coulometric detection-are they reliable biomarkers of oxidative stress? Anal Biochem. 1995; 229:329–335.
8. Iriyama K, Yoshiura M, Iwamoto T, Ozaki Y. Simultaneous determination of uric and ascorbic acids in human serum by reversed-phase high-performance liquid chromatography with electrochemical detection. Anal Biochem. 1984; 141:238–243.

9. Khan A, Khan MI, Iqbal Z, Shah Y, Ahmad L, Nazir S, et al. A new HPLC method for the simultaneous determination of ascorbic acid and aminothi-ols in human plasma and erythrocytes using electrochemical detection. Talanta. 2011; 84:789–801. doi: 10.1016/j.talanta.2011.02.019.

10. Li X, Franke AA. Fast HPLC-ECD analysis of ascorbic acid, dehydroascorbic acid and uric acid. J Chromatogr B Analyt Technol Biomed Life Sci. 2009; 877:853–856. doi: 10.1016/j.jchromb.2009.02.008.

11. Watson DG, Iqbal Z, Midgley JM, Pryce-Jones H, Morrison L, Dutton GN, et al. Measurement of ascorbic acid in human aqueous humour and plasma and bovine aqueous humour by high-performance liquid chromatography with electrochemical detection. J Pharm Biomed Anal. 1993; 11:389–392.

12. Schell DA, Bode AM. Measurement of ascorbic acid and dehydroascorbic acid in mammalian tissue utilizing HPLC and electrochemical detection. Biomed Chromatogr. 1993; 7:267–272.

13. Umegaki K, Inoue K, Takeuchi N, Higuchi M. Improved method for the analysis of ascorbic acid in plasma by high-performance liquid chromatography with electrochemical detection. J Nutr Sci Vitaminol (Tokyo). 1994; 40:73–79.

14. Margolis SA, Davis TP. Stabilization of ascorbic acid in human plasma, and its liquid-chromatographic measurement. Clin Chem. 1988; 34:2217–2223.

15. Mannino S, Cosio MS. Determination of ascorbic acid in foodstuffs by microdialysis sampling and liquid chromatography with electrochemical detection. Analyst. 1997; 122:1153–1154.

16. Nagy E, Degrell I. Determination of ascorbic acid and dehydroascorbic acid in plasma and cerebrospinal fluid by liquid chromatography with electrochemical detection. J Chromatogr. 1989; 497:276–281.

17. Karlsen A, Blomhoff R, Gundersen TE. High-throughput analysis of vitamin C in human plasma with the use of HPLC with monolithic column and UV-detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2005; 824:132–138.

18. Burini G. Development of a quantitative method for the analysis of total L-ascorbic acid in foods by high-performance liquid chromatography. J Chromatogr A. 2007; 1154:97–102.

19. Guidance for industry: bioanalytical method validation. In. Edited by U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research (CDER): Center for Biologics Evaluation and Research (CBER); May. 2001.
20. Melethil S, Mason WD, Chang CJ. Dose-dependent absorption and excretion of vitamin C in humans. Int J Pharm. 1986; 31:83–89.

21. Dhariwal KR, Washko PW, Levine M. Determination of dehydroascorbic acid using high-performance liquid chromatography with coulometric electrochemical detection. Anal Biochem. 1990; 189:18–23.

22. Kim YJ, Ha N, Kim MG. Simultaneous determination of L-ascorbic acid and dehydroascorbic acid in human plasma. Analytical Methods. 2015; 7:9206–9210.
Figure 1.
Chemical structure of ascorbic acid and dehydroascorbic acid as depicted through a redox reaction
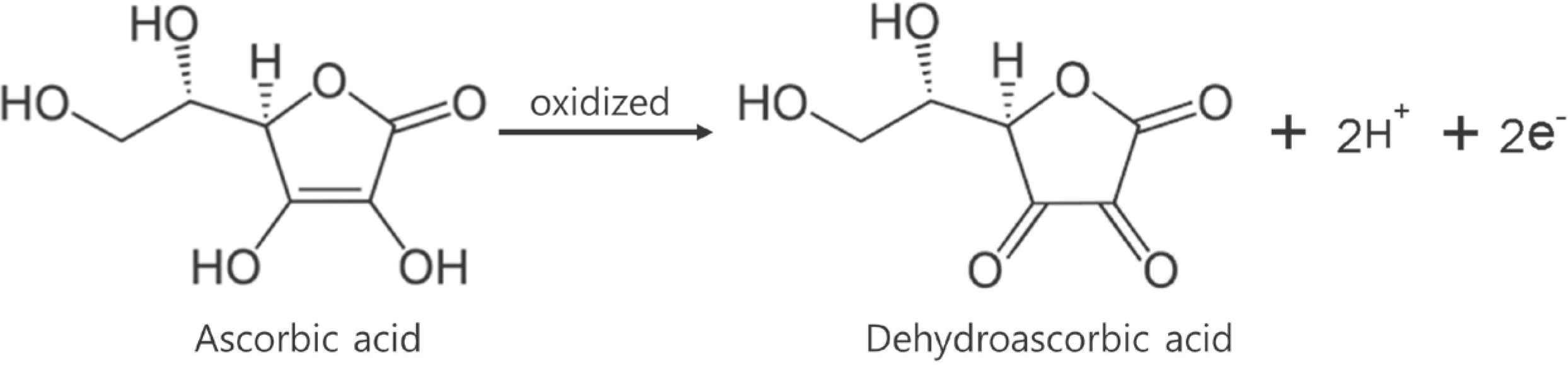
Figure 2.
Recovered ascorbic acid contents in standard solutions stored at −70°C (■), −4°C (▲), 25°C (◆) and light shielded at 25°C (●)
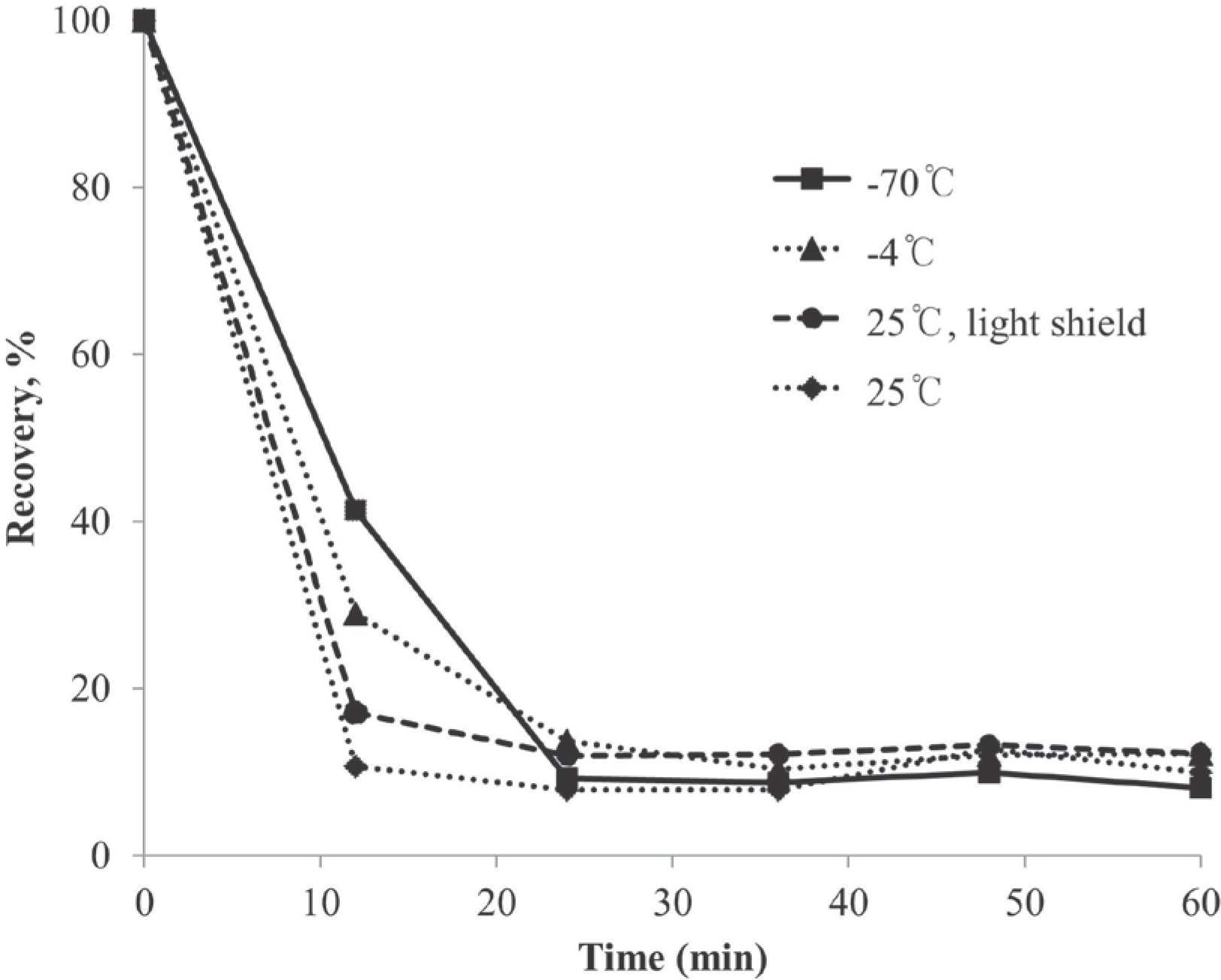
Figure 3.
Chromatogram of ascorbic acid and internal standards in human plasma. HPLC conditions: mobile phase, 5 mM HTAB + 50 mM KH2PO4; column, C18 column (symmetry® 4.6 μm × 280 mm, 5 μm particle size, Waters, USA); flow rate, 1.2 mL/min; UV detection wavelength, 254 and 265 nm.
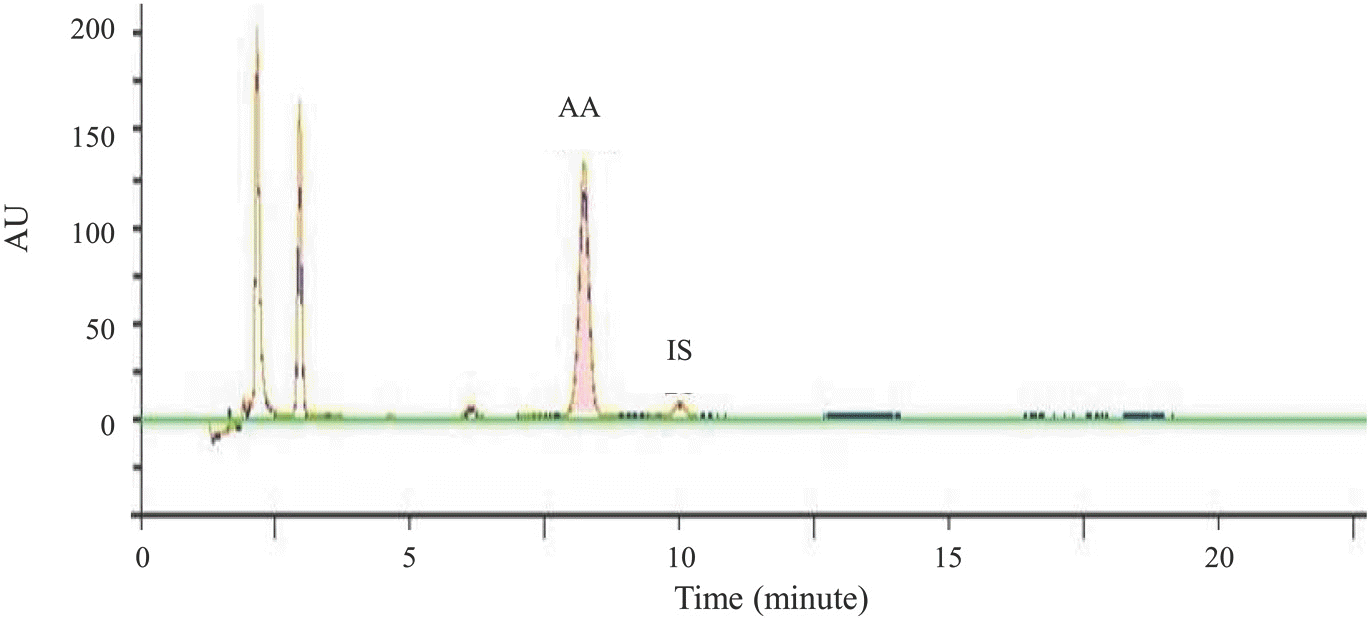
Table 1.
Intra- and inter-day precision and accuracy (n=5) of ascorbic acid QC samples in human plasma
Table 2.
Intra- and inter-day recovery of ascorbic acid in human blank plasma QC samples (n=5)
| Concentration (μg/mL) | Recovery (%, Mean±S.D) | |
|---|---|---|
| Intra-day | Inter-day | |
| 1 | 106.00±3.20 | 98.98±6.52 |
| 2 | 108.30±4.39 | 110.05±12.84 |
| 50 | 118.41±3.44 | 109.41±8.93 |
| 80 | 100.89±2.69 | 104.01±2.72 |
Table 3.
Dehydroascorbic acid concentrations
| Free AA (μg/mL) | Total AA (μg/mL) | DHAA |
|---|---|---|
| AA 50 μg/mL | AA 50 μg/mL + 2 mM DTT | Total AA – free AA |
| 37.06 | 44.24 | 7.18 |
Table 4.
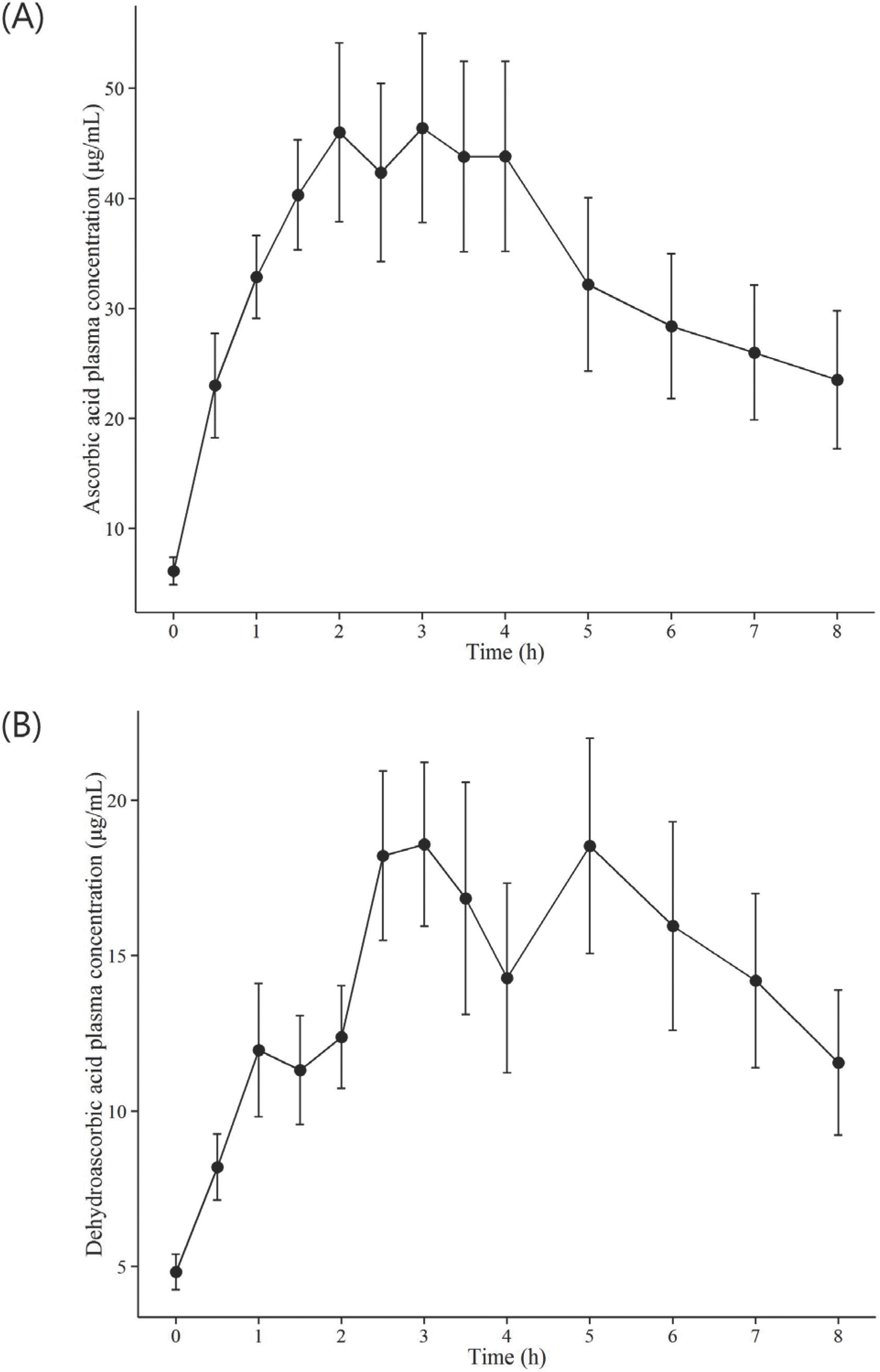
Pharmacokinetic parameters for ascorbic acid and dehydroascorbic acid in human plasma (mean±SD) obtained after administration of vitamin C 4,000 mg




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download