Abstract
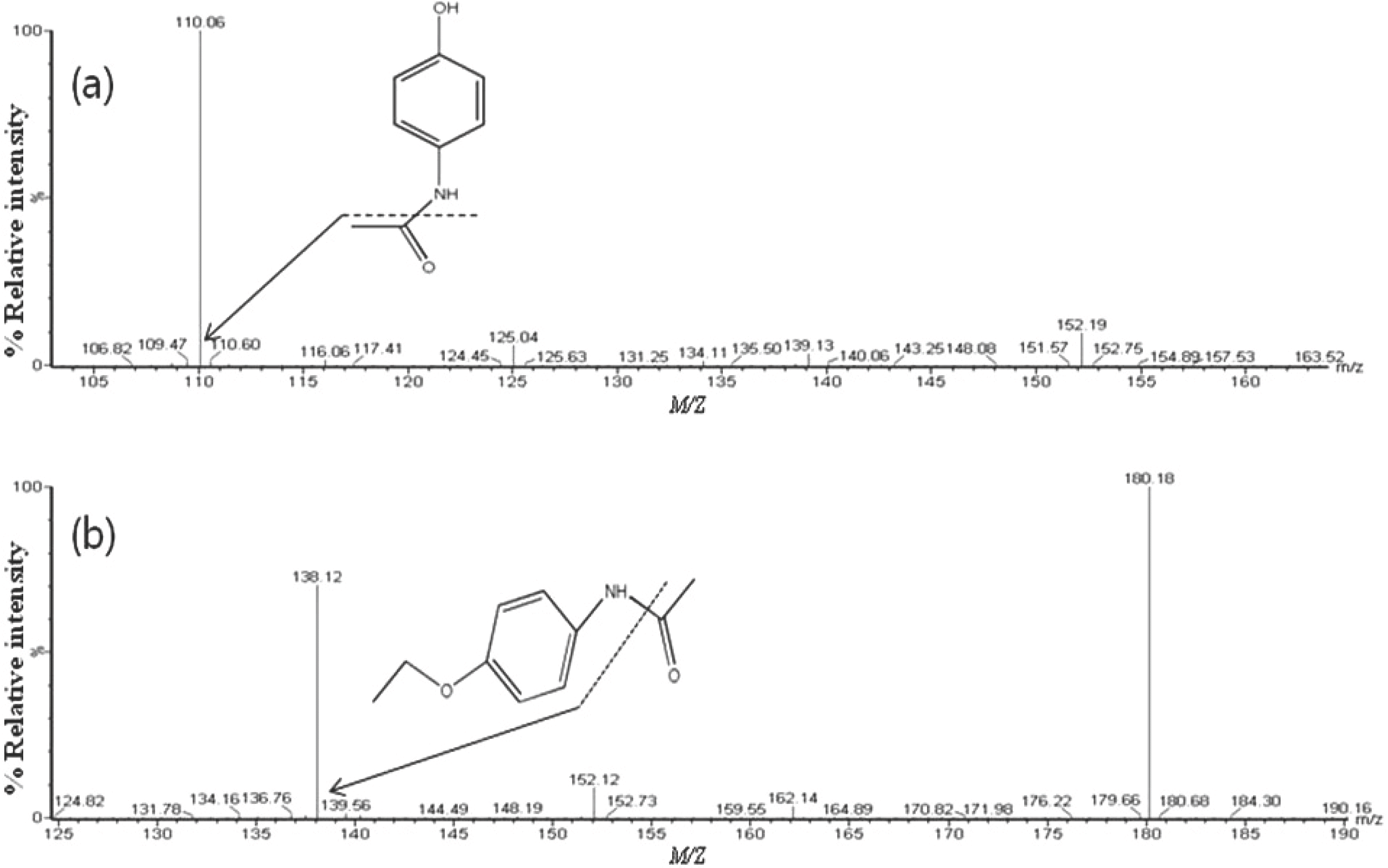
We developed an ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method for the determination of acetaminophen concentration in human plasma. Following protein precipitated extraction, the analytes were separated and analyzed using an UPLC-MS/MS in the multiple reaction monitoring (MRM) mode with the respective [M+H]+ ions, m/z 152.06 → 110.16 for acetaminophen and m/z 180.18 → 138.12 for phenacetin (internal standard, IS). The method showed a linear response from 1 to 100 µg/mL (r > 0.9982). The limit of quantitation for acetaminophen in plasma was 1 µg/mL. The intra- and inter-day accuracy ranged in the ranges of 94.40–99.56% and 90.00–99.20%, respectively. The intra- and inter-day precision ranged in the ranges of 2.64–10.76% and 6.84–15.83%, respectively. This method was simple, reliable, precise and accurate and can be used to determine the concentration of acetaminophen in human plasma. Finally, this fully validated method was successfully applied to a pharmacokinetic study of acetaminophen in healthy volunteers following oral administration.
References
1. Smith HS. Perioperative Intravenous Acetaminophen and NSAIDs. Pain Med. 2011; 12:961–981. doi: 10.1111/j.1526-4637.2011.01141.x.

2. Perrott DA, Piira T, Goodenough B, Champion GD. Efficacy and safety of acetaminophen vs ibuprofen for treating children's pain or fever: a metaanalysis. Arch Pediatr Adolesc Med. 2004; 158:521–526.
3. Yin OQ, Tomlinson B, Chow AH, Chow MS. Pharmacokinetics of acetaminophen in Hong Kong Chinese subjects. Int J Pharm. 2001; 222:305–308.

4. Flores-Pérez C, Chávez-Pacheco JL, Ramírez-Mendiola B, Alemón-Medina R, García-Álvarez R, Juárez-Olguín H, et al. A reliable method of liquid chromatography for the quantification of acetaminophen and identification of its toxic metabolite N-acetyl-p-benzoquinoneimine for application in pediatric studies. Biomed Chromatogr. 2010; 25:760–766. doi: 10.1002/bmc. 1511.

5. Abu-Qare AW, Abou-Donia MB. A validated HPLC method for the determination of pyridostigmine bromide, acetaminophen, acetylsalicylic acid and caffeine in rat plasma and urine. J Pharm Biomed Anal. 2001; 26:939–947.

6. Alkharfy KM, Frye RF. High-performance liquid chromatographic assay for acetaminophen glucuronide in human liver microsomes. J Chromatogr B Biomed Sci Appl. 2001; 753:303–308.

7. Brunner LJ, Bai S. Simple and rapid assay for acetaminophen and conjugated metabolites in low-volume serum samples. J Chromatogr B Biomed Sci Appl. 1999; 732:323–329.

8. Lufeng Hu, Xuezhi Yang, Xianqin Wang, Jiayin Zhu, Shuhua Tong, Gaozhong Cao. Rapid LC-APCI-MS-MS method for simultaneous determination of phenacetin and its metabolite paracetamol in rabbit plasma. Chromatophia. 2009; 70:585–590.
9. Zhang Y, Mehrotra N, Budha NR, Christensen ML, Meibohm B. A tandem mass spectrometry assay for the simultaneous determination of acetaminophen, caffeine, phenytoin, ranitidine, and theophylline in small volume pediatric plasma specimens. Clin Chim Acta. 2008; 398:105–112. doi: 10.1016/j.cca.2008.08.023.

10. Johnson KA, Plumb R. Investigating the human metabolism of acetaminophen using UPLC and exact mass oa-TOF MS. J Pharm Biomed Anal. 2005; 39:805–810.

11. Sun J, Schnackenberg LK, Holland RD, Schmitt TC, Cantor GH, Dragan YP, et al. Metabonomics evaluation of urine from rats given acute and chronic doses of acetaminophen using NMR and UPLC/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008; 871:328–340. doi: 10.1016/j.jchromb.2008.04.008.

12. Marin A, Garcia E, Garcia A, Barbas C. Validation of a HPLC quantification of acetaminophen, phenylephrine and chlorpheniramine in pharmaceutical formulations: capsules and sachets. J Pharm Biomed Anal. 2002; 29:701–714.
13. Nováková L, Matysová L, Solich P. Advantages of application of UPLC in pharmaceutical analysis. Talanta. 2006; 68:908–918. doi: 10.1016/j.talanta.2005.06.035.

14. Phapale PB, Lee HW, Lim MS, Kim EH, Kim SD, Park J, et al. Rapid determination of finasteride in human plasma by UPLC-MS/MS and its application to clinical pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2008; 878:1718–1723. doi: 10.1016/j.jchromb.2010.04.029.

15. Chen X, Huang J, Kong Z, Zhong D. Sensitive liquid chromatography tandem mass spectrometry method for the simultaneous determination of paracetamol and guaifenesin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2005; 817:263–269.
16. Wren SA, Tchelitcheff P. Use of ultra-performance liquid chromatography in pharmaceutical development. J Chromatogr A. 2006; 1119:140–146.

17. Behnoush B, Sheikhazadi A, Bazmi E, Fattahi A, Sheikhazadi E, Saberi Anary SH. Comparison of UHPLC and HPLC in benzodiazepines analysis of postmortem samples: a case-control study. Medicine (Baltimore). 2015; 94:e640. doi: 10.1097/MD.0000000000000640.
18. Ni KH, Wen ZD, Huang XC, Wang CX, Ye TT, Hu GX, et al. Determination of trifolirhizin in rat plasma by UPLC: Application to apharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2015; 990:181–184. doi: 10.1016/j.jchromb.2015.03.031.
19. Tonoli D, Varesio E, Hopfgartner G. Quantification of acetaminophen and two of its metabolites in human plasma by ultrahigh performance liquid chromatography–low and high resolution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2012; 904:42–50. doi: 10.1016/j.jchromb.2012.07.009.

20. Qiu X, Lou D, Su D, Liu Z, Gao P, Zhang NS. Simultaneous determination of acetaminophen and dihydrocodeine in human plasma by UPLC-MS/MS: Its pharmacokinetic application. J Chromatogr B Analyt Technol Biomed Life Sci. 2015; 992:91–95. doi: 10.1016/j.jchromb.2015.04.031.

21. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinfor-mation/guidances/ucm070107.pdf. Accessed 17August. 2015.
22. http://www.mfds.go.kr/index.do?mid=1161&seq=7560. Accessed 17August. 2015.
23. Palma-Aguirre JA, Villalpando-Hernández J, Novoa-Heckel G, Oliva I, Cariño L, López-Bojórquez E, et al. Bioavailability of two oral-tablet and two oral-suspension formulations of naproxen sodium/paracetamol (acetaminophen): single-dose, randomized, open-label, two-period crossover comparisons in healthy Mexican adult subjects. Clin Ther. 2009; 31:399–410. doi: 10.1016/j.clinthera.2009.02.002.

Figure 2.
Typical MRM chromatograms of acetaminophen (upper panel) and phenacetin (lower panel) in human plasma samples obtained from (a) a drug-free blank plasma sample, (b) a plasma sample spiked with acetaminophen at the LLOQ (1 μg/mL) and 1 μg/mL phenacetin and (c) plasma from a volunteer 1 h after oral administration of a 1,000 mg dose of acetaminophen spiked with phenacetin (experimental conditions were same as in text).
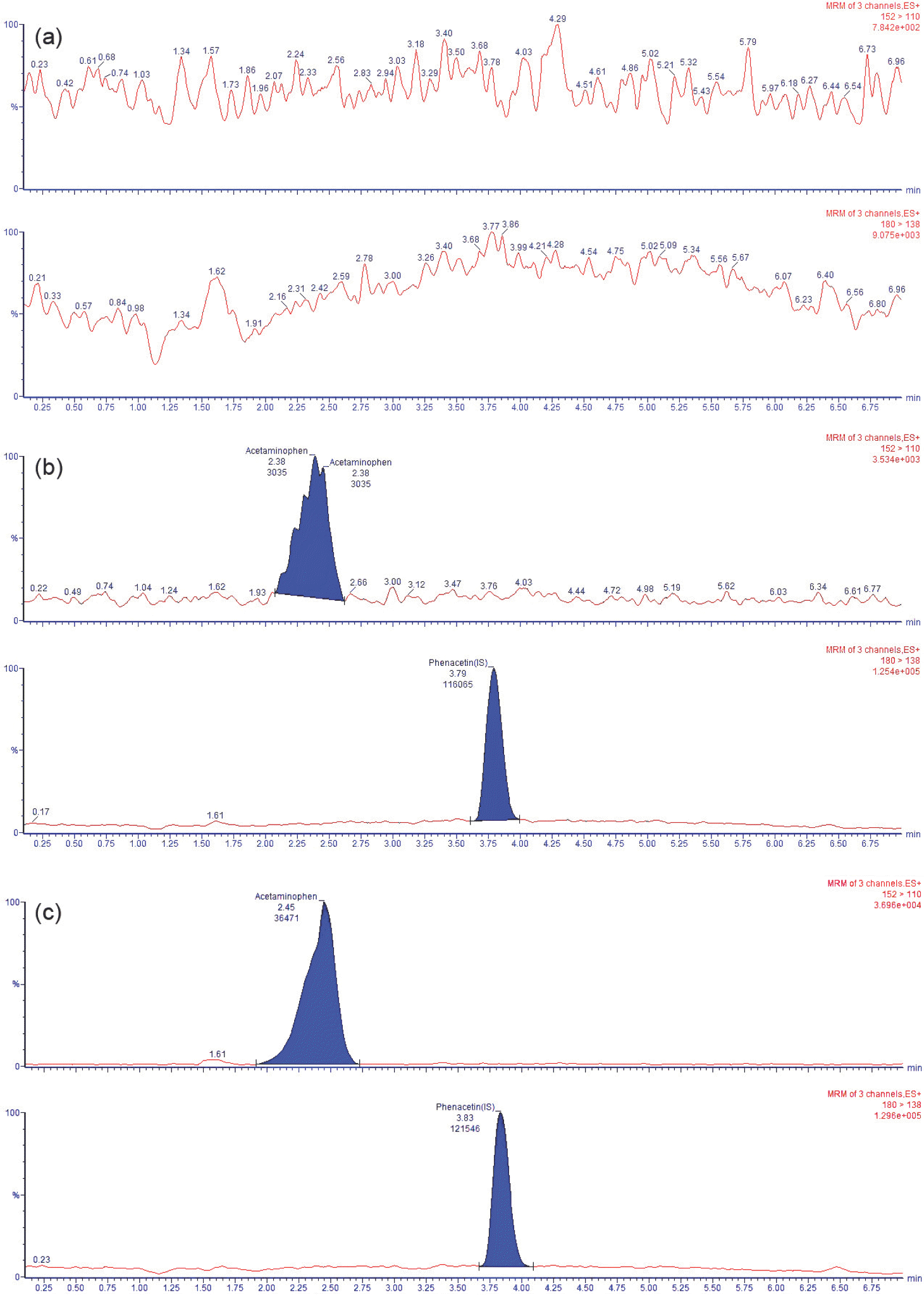
Figure 4.
Mean plasma concentration-time profile of acetaminophen after a 1,000 mg oral dose in 8 healthy volunteers. The vertical bars show the standard errors.
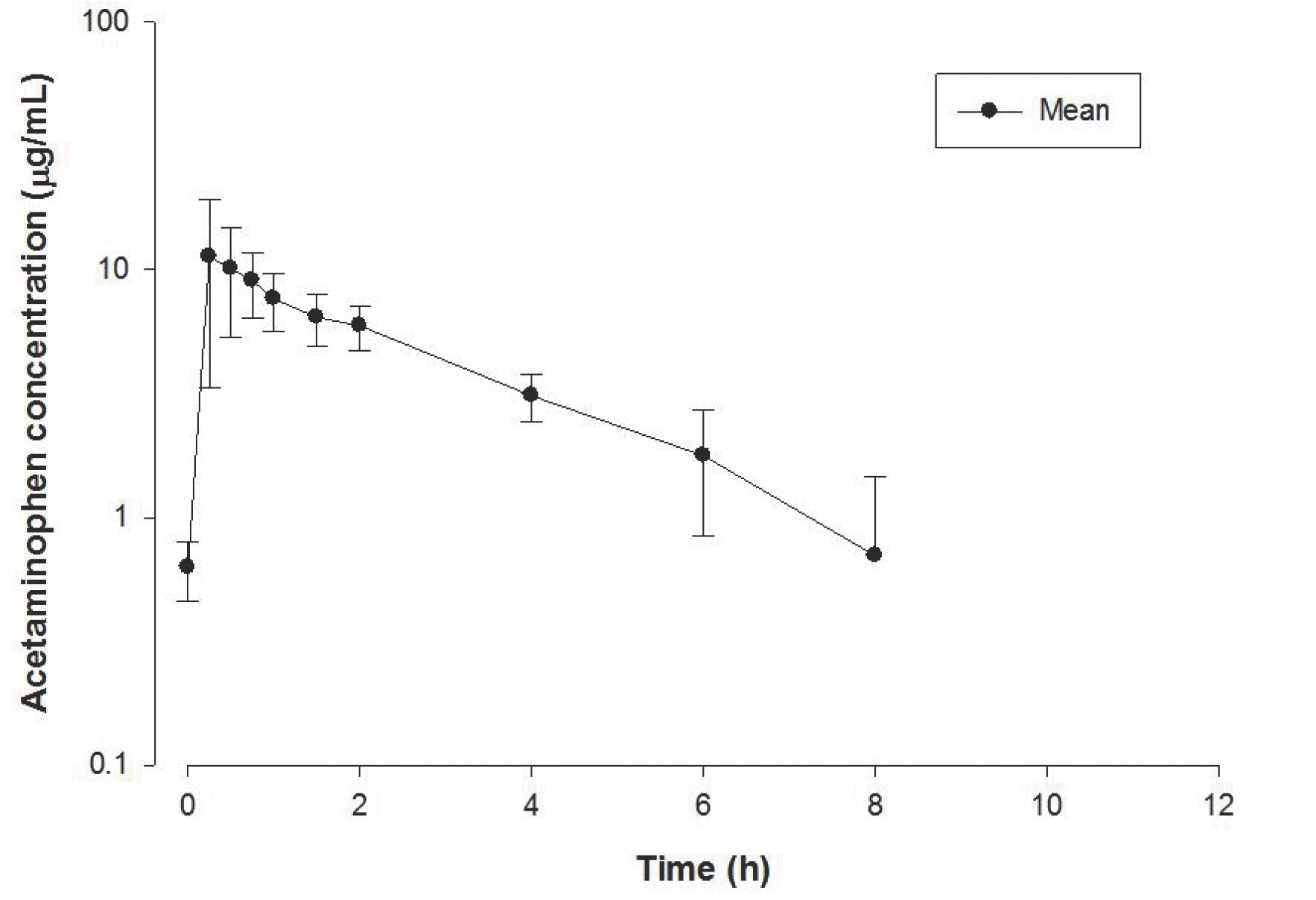
Table 1.
Recovery of acetaminophen preparation and extraction
| Nominal concentrations (μg/mL) | Recovery (mean±SDa, %) | RSDb (%) |
|---|---|---|
| Acetaminophen | ||
| 2 | 91.70±0.69 | 8.26 |
| 50 | 89.40±0.16 | 1.59 |
| 80 | 89.00±0.17 | 1.56 |
| 10 | Phenacetine ((ISc) | |
| 10.25±0.34 | 3.30 | |
Table 2.
The intra- and inter-day precision and accuracy of quality-control samples containing acetaminophen at four concentrations (1, 2, 50, and 80 mg/mL) in plasma (weight: 1/x)
| Nominal concentration (μg/mL) | Intra-day (n = 5) | Inter-day (n = 5) | ||
|---|---|---|---|---|
| Accuracy (%) | Precision (%RSDa) | Accuracy (%) | Precision (%RSD) | |
| 1 | 96.00 | 10.76 | 90.00 | 15.83 |
| 2 | 99.00 | 7.06 | 95.00 | 8.15 |
| 50 | 99.56 | 3.04 | 99.20 | 6.84 |
| 80 | 94.40 | 2.64 | 96.53 | 8.44 |
Table 3.
Stability of acetaminophen under four different conditions (n = 3)
| Storage condition | LQCa (2 μg/mL) | HQCb (80 μg/mL) | |||
|---|---|---|---|---|---|
| Testc | Referenced | Test | Reference | ||
| Post-preparation | Mean (mg/mL) | 2.00 | 2.07 | 85.63 | 75.70 |
| SDe (±) | 0.20 | 0.12 | 1.86 | 4.36 | |
| RSDf (%) | 10.00 | 5.60 | 2.20 | 5.80 | |
| Freeze-thaw cycles (for 3 cycles) | Mean (mg/mL) | 1.73 | 1.83 | 66.77 | 75.83 |
| SD (±) | 0.21 | 0.23 | 3.03 | 0.99 | |
| RSD (%) | 12.00 | 12.60 | 4.50 | 1.30 | |
| Short-term | Mean (mg/mL) | 2.20 | 2.07 | 79.60 | 82.97 |
| SD (±) | 0.00 | 0.23 | 1.22 | 3.04 | |
| RSD (%) | 0.00 | 11.200 | 1.50 | 3.70 | |
| Long-term | Mean (mg/mL) | 2.60 | 2.40 | 76.47 | 81.03 |
| SD (±) | 0.10 | 0.27 | 3.79 | 4.02 | |
| RSD (%) | 3.80 | 11.00 | 5.00 | 5.00 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


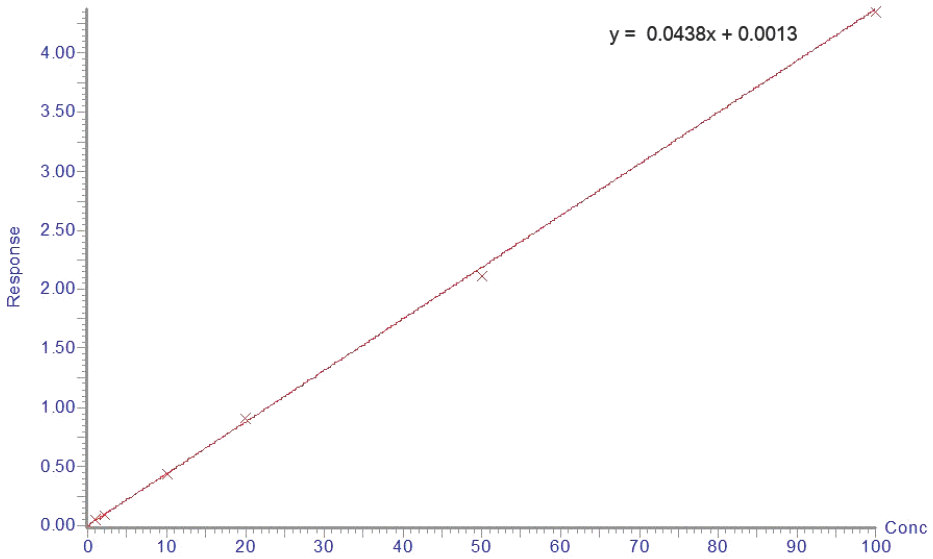
 XML Download
XML Download